1. Introduction
PNETs, a set of endocrine tumors originating in the pancreas, are considered one of the most common types of NETs. However, classified as one of the pancreatic cancers, only less than 2% of the patients are diagnosed as PNETs. This is because most of the pancreatic cancers start in the exocrine cells instead of the endocrine cells [1]. NETs have shown a significant surge in most of the developed countries. According to the data given, it is estimated to be around 1.01–5.25 cases per 100 000 population, which is becoming more and more influential in a negative way [2].
Improvements in imaging technology and the knowledge of these tumors have made it easier to diagnose PNETs that are asymptomatic or symptomatic. To diagnose a pancreatic NET accurately, a patient should undergo the following tests and procedures: blood tests, urine tests, imaging tests (e.g. X-ray, MRI, CT, PET scan), producing internal pictures of the pancreas, doing a biopsy to remove a sample of tissue for analysis, and gathering cells from other regions to examine whether the cancer has spread elsewhere [3,4]. Current approved standard treatments for pancreatic NETs have limited choice, including surgery, specific functional PNET treatments, chemotherapy, peptide receptor radionuclide therapy, and tumor ablation .
Immune checkpoint inhibitors (ICIs) as one of the most effective immunotherapies, is not approved as a standard treatment for the PNETs. Aiming to inhibit tumor growth and destruction directly, traditional cancer treatments, such as radiotherapy, chemotherapy, and targeted agents still have a long way to go before reaching the effectiveness of immunotherapies. The sustained effectiveness of these direct-acting medicines is frequently restricted by resistance mechanisms and toxicities. In contrast, immunotherapies work with the patient's immune system and make it well-equipped to fight cancer [5,6]. If it is possible for ICI approved for PNETs treatment, not only will the patients gain more choices, but also a decrease in mortality rate of PNETs.
This review will provide a comprehensive analysis of whether ICIs are effective for treating PNETs, and problems and improvements of ICIs towards PNETs. Starting with the mechanism of ICIs, including PD-1 and CTLA-4, a clear explanation of this immunotherapy is given for a better understanding of this paper. There will be further discussion on the application of ICIs including negative responses from patients and drug resistance. Improvements on the ICIs about combination and personalized treatments are also deliberated.
2. Mechanism of ICIs
Throughout the years, the development in immunotherapy has shown promising results, especially in ICIs, where several inhibitory immunoreceptors, such as PD-1, CTLA-4, LAG3, TIM3, TIGIT, and BTLA, have been identified and further investigated in relation to cancer [7]. These receptors frequently deliver inhibitory signals via mono-tyrosine signaling pathways, such as ITSM and ITIM. Since they are surface molecules, blocking antibodies that obstruct ligand-receptor contact is an easy way to reduce their activity [8]. However, only PD-1 and CTLA-4 are extensively studied and approved by the FDA. Thus, the most broadly used in treating cancer around the globe in terms of ICIs.
Originally, the immune system checkpoints are designed to avert the immune response from being too overactive or exaggerated and accidentally harm healthy cells. The specific activation of tumor antigen T cell receptor and MHC connection allows T cells to identify cancerous cells. In normal cases, an immune response will be triggered and the T cell will release cytokines to eliminate the tumor cell whenever the TCR bind to the antigen of cancer cell. When the complementary proteins and checkpoint bind together, T cells get a "shut down" sign. This may prevent the immune system from destroying the malignancy by deactivating the T cell [9]. In order to avoid being killed, cancer cells usually express a high level of immune checkpoint proteins. This leads to a higher chance of binding with the receptor on T cells, causing it to be turned off. Hence, fewer cancer cells are being destroyed.
The most important discovery in cancer treatment over the last 10 years has been the introduction of T cell-focused immunomodulators that block the immunological checkpoints CTLA-4 and PD-1/PD-L1. Ipilimumab, the first antibody to suppress CTLA-4, received FDA approval in 2011. Soon after this, new types of monoclonal antibodies that aim at PD-L1 and PD-1 followed. Anti-PD-L1 includes atezolizumab and durvalumab, and anti PD-1 includes pembrolizumab and nivolumab. The two abovementioned antibodies are now among the anticancer medications that are prescribed the most frequently. These days, T-cell-targeted immunomodulators are used either alone or in combination with chemotherapy as first or second lines of treatment for about 50 distinct forms of cancer. Approximately two-thirds of all clinical trials in cancer are now underway, with over 3000 trials assessing T cell modulators [10].
2.1. PD-1
T cells, B cells, and NK cells are immune cells that express the cell surface receptor PD-1. Because it modifies T-cell activity, induces antigen-specific T cell apoptosis, and inhibits regulatory T cell apoptosis, PD-1 is crucial for reducing immunological responses and promoting self-tolerance [11]. When PD-1 attaches to its ligand, PD-L1, T cell activation is inhibited and downstream signaling pathways are activated [12]. As a transmembrane molecule, PD-L1 is also considered to be a co-suppressive factor in immunity. By inhibiting PD-L1, the growth of certain cells that release cytokines to destroy cancer cells can be hindered. (Fig.1) However, it happens only when combined with blocking PD-1. Certain tumors can evade the immune system's identification thanks to PD-L1, and inhibiting PD-L1 enhances the immune system's capacity to identify and combat various cancer types. Cancer is able to evade host immunity through the immune checkpoint signaling pathway known as the PD-1/PD-L1 axis [11].
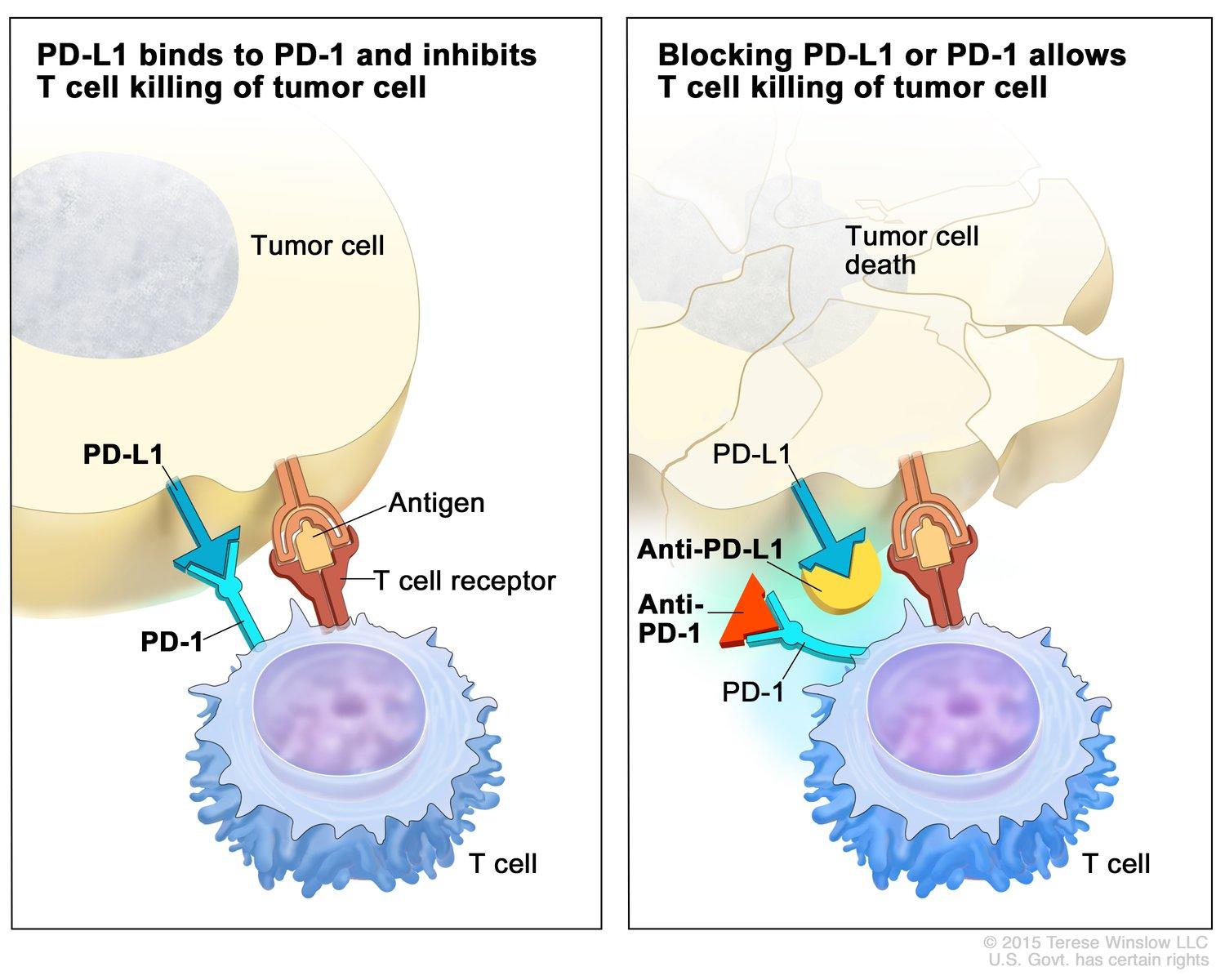
Figure 1. The left-hand side shows the release of cytokines of T cell is inhibited by the binding of PD-1 and PD-L1. The right-hand side shows through the binding with anti-PD-1 and anti-PD-L1, the T cell is able to destroy the tumor cell [13].
2.2. CTLA-4
CTLA-4 is the other unfavorable modulator of T cell immunity. It is thought to regulate T-cell proliferation early in an immunological response, primarily in lymph nodes [14]. Both CD4 and CD8 positive cells frequently express CTLA-4, which binds to T-cell-costimulatory factors on the surface of APC. CTLA-4 binding inhibits T-cell proliferation and the generation of IL-2 [15]. The inhibitory molecule CTLA-4, often referred to as CD152, is found on the surface of T cells that have been activated, and it keeps B7 from attaching to CD28. Its mechanism reduces T cell responses by stopping the first phase of naive T cell stimulation in the lymph nodes. The purpose of anti-CTLA-4 antibodies is to decrease T cell activity by preventing CTLA-4 binding [16].
3. Application of ICIs on PNETs
3.1. Drug
Treatment management for PNETs is extremely challenging as most of them only shows little efficacy. NETs are a subtype of neuroendocrine neoplasms (NENs) that are generally well-differentiated and low-proliferating [17]. NETs usually show low tumor mutation burden (TMB).
However, in terms of treatments targeting PNETs, there is no specific FDA or other regulatory approval for the use of either pembrolizumab, nivolumab, ipilimumab, etc. Hence, the data of the use of these ICIs are limited and only based on small studies or case reports.
From the KEYNOTE-028 study, only 16 patients had PD-L1-positive tumor among 106patients. It is evaluated that the use of pembrolizumab in the 16 PNETs patients only shows 6.3% objective response rate (ORR), but early response and durable, which is significant given the advanced, heavily pretreated nature of the patients [18].
Data from PanNEN phase I/II clinical trials of different ICIs have been widely collected. T Phase Ib data on the PD-1 inhibitor tirapalimab showed an ORR of 22.2% among the PanNEN subgroup, which is composed of patients with positive PD-L1, TMB-H, or MSI-H status. Yet, no significant benefit between ICI monotherapy and NENs in newly conducted trials [19]. ICIs treatments show limited efficacy on PanNENs. Since ICI monotherapies appear to have little anticancer effect, dual ICI treatments have been employed in the clinic to boost clinical efficacy. From the DART phase II clinical trial, 44% of patients with high-grade NETs experienced tumor shrinkage (full or partial response) after receiving ipilimumab and nivolumab together. On the other hand, patients with low-grade NETs did not show any decrease in size of the tumor [20]. PNET patients may benefit from this pharmaceutical application regimen more than non-PNET patients due to the 20% ORR that comes with the combination of targeted anti-angiogenesis and ICI therapy [21]. It can be concluded that combined treatments show higher efficacy in PNETs. Overall saying, the application of ICI on PNETs is still undergoing investigation and the information provided by those small-scale researches is not enough to make a definite conclusion on the effectiveness of the application of ICI.
3.2. Adverse events
In KEYNOTE-028 study, 94% of the patient experienced adverse events (AEs). The most frequent side effects associated with pembrolizumab therapy were fatigue and diarrhea, but the PNET cohort did not experience any discontinuations due to treatment-related adverse events. One patient in the PNET cohort had malaise and grade 2 dyspnea. There were few treatment-related grade 3 adverse events, and neither cohort experienced any grade 4 adverse events or mortality [18]. In DART study, where patients are treated with ipilimumab and nivolumab together, the two most prevalent toxicities overall were nausea, and fatigue. Aspartate transaminase increase and hypothyroidism were the most common immune-related toxicities of any grade. Abnormal liver function and colitis, among other immune-related toxicities, were the most common grade 3 toxicities. However, both deadly toxicity and immune-related pneumonitis instances were absent [20]. Overall, fatigue is the most common adverse events of PNET treatments. Although there are various adverse events that may affect the patients negatively, all treatments have undergone safety control that all approved treatments only show some minor ones and will not threaten the lives of patients.
3.3. Drug resistance
As combination therapies often shows a higher efficacy towards PNETs, it can be hypothesized that monotherapies solely may not be able to overcome the drug resistance [22]. Therefore, combination therapies including two ICIs, ICIs with target anti-angiogenesis, or ICIs with chemotherapy are necessary in treating PNETs more efficiently. Proved in many studies, combinatorial therapies give maximum clinical impact. In order to develop more successful treatment strategies, there is the need to understand the immunological features and tumor microenvironment of PNETs, where the tumor microenvironment may play a role in promoting drug resistance mechanisms in this type of disease [22]. Since a pathogen or tumor is less likely to exhibit resistance to many medications at once, combination treatments have the major benefit of reducing the development of drug resistance.
4. Optimization
4.1. Personalization
Optimizing treatment outcomes necessitates personalizing cancer immunotherapy by genetic analysis, biomarker identification, and T-cell dynamics monitoring. By identifying specific biomarkers, the response of patients to ICIs become predictable, which is a crucial part for personalizing treatment. For example, higher levels of CD8+ T-cell infiltration correlate directly with better responses to ICI therapy. While PD-L1 expression also shows some positive correlation with the responses, it is not always consistent [23]. In terms of genomic and transcriptomic profiling, high TMB and high MSI have shown a higher probability to respond to ICIs, leading to better outcomes. Mutational analysis is also vital in ICI therapy by identifying somatic mutations and understanding resistance mechanisms. By finding mutations that create an ultramutator phenotype in specific genes, such as POLE and POLD1, it may be feasible to identify individuals who could respond well to therapy. Moreover, if more scientific researches are done on mutations that lead to resistance (e.g., in PTEN, EGFR, STK11), the choice of combination therapies or alternative treatments to improve patient outcomes could have a clear path [23]. All of these aforementioned can help personalize treatment decisions. By evaluating them, clinicians can identify and prioritize patients who are more likely to benefit from immunotherapy, leading to a more effective treatment strategy.
4.2. Combination therapy
Combination therapy often shows higher efficacy. ICIs, especially, have the flexibility to combine with many other therapies. For instance, two different types of ICIs, chemotherapy, targeted therapy, anti-angiogenic agent, cancer vaccine, radiation therapy, etc. However, treatments for PNET using combination therapy are not widely studied yet, only dual ICIs, chemotherapy, and targeted therapy are studied in small scale. As dual ICIs are discussed previously, it is not going to be mentioned again here. For ICI combined with targeted anti-angiogenic agent, the combination of atezolizumab (an ICI) and bevacizumab (an anti-angiogenic agent) in patients with grade 1/2 PNETs and non-PNETs are studied. Resulting with a 20% ORR for both and 1-Year PFS rates of 75% for PNETs and 52% for non-PNETs, PNET patients may benefit more from this combination therapy [23]. In a Phase II study of ICI combined with chemotherapy, 12 out of 15 patients with advanced NETs were treated with nivolumab and temozolomide. Outcomes showed 25% achieved PR, 67% had SD, while one patient had PD [21]. This indicates potential effectiveness of this regimen in advanced NETs.
5. Conclusion
This review has discussed the application of ICIs on PNETs--from the mechanism of ICIs to the personalized treatment and combination therapy for PNETs. Up till this moment, there is no ICI therapies approved by the FDA specifically for PNET as the efficacy is too low. While ICIs have shown promising result in treating other types of NETs, particularly in small cell lung cancer and melanoma, their role in PNETs remains less indispensable. Yet, combination therapy shows relatively higher efficacy in treating PNETs since they are more able to overcome drug resistance. As mentioned in the introduction, PNET is a rare type of NET and pancreatic cancer, hence, not much studies are done. Since the effectiveness of ICIs treatments on PNETs are not significant, ICIs could never be one of the common treatments for PNETs until better outcomes are seen or new checkpoints discovered. However, it is crucial to recognize this review's limits. The long-term effects of combination therapies and the molecular predictors of response to ICI remain underexplored. Additionally, our analysis did not extensively cover the role of the tumor microenvironment, which could greatly influence treatment efficacy and patient prognosis. Looking ahead, research in the future should concentrate on finding biomarkers for patient classification and conducting long-term studies to evaluate the efficacy of combination medicines. enhance patient outcomes in the end and open the door for novel PNET treatment modalities.
References
[1]. American Cancer Society nd What is a pancreatic neuroendocrine tumor? Retrieved August 7 2024 from: https://www.cancer.org/cancer/types/pancreatic-neuroendocrine-tumor/about/what-is-pnet.html
[2]. Gubbi S Vijayvergia N Yu J Q Klubo-Gwiezdzinska J & Koch C A 2022 Immune checkpoint inhibitor therapy in neuroendocrine tumors Hormone and Metabolic Research 54 12 795
[3]. Panda A Garg I Johnson G B Truty M J Halfdanarson T R & Goenka A H 2019 Molecular radionuclide imaging of pancreatic neoplasms The Lancet Gastroenterology & Hepatology 4 7 559–570
[4]. Kendi A T Halfdanarson T R Packard A Dundar A & Subramaniam R M 2019 Therapy with 177Lu-DOTATATE: Clinical implementation and impact on care of patients with neuroendocrine tumors American Journal of Roentgenology 213 2 309–317
[5]. Hopper A D Jalal M & Munir A 2019 Recent advances in the diagnosis and management of pancreatic neuroendocrine tumours Frontline Gastroenterology 10 3 269–274
[6]. Pennock G K & Chow L Q M 2015 The evolving role of immune checkpoint inhibitors in cancer treatment The Oncologist 20 7 812–822
[7]. Zhang H Dai Z Wu W Wang Z Zhang N Zhang L & He X 2021 Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer Journal of Experimental & Clinical Cancer Research 40 1 184
[8]. He X & Xu C 2020 Immune checkpoint signaling and cancer immunotherapy Cell Research 30 8 660–669
[9]. Gomatou G Tzilas V Kotteas E Syrigos K & Bouros D 2020 Immune checkpoint inhibitor-related pneumonitis Respiration 99 11 932–942
[10]. Nature Communications 2020 A decade of immune-checkpoint inhibitors in cancer therapy Nature Communications
[11]. Han Y Liu D & Li L 2020 PD-1/PD-L1 pathway: Current researches in cancer American Journal of Cancer Research 10 3 727–742
[12]. Jiang Y Chen M Nie H & Yuan Y 2019 PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations Human Vaccines & Immunotherapeutics 15 5 1111–1122
[13]. Immune Checkpoint Inhibitors - NCI. 24 Sept. 2019, https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors. nciglobal, ncienterprise.
[14]. Buchbinder E I & Desai A 2016 CTLA-4 and PD-1 pathways: Similarities differences and implications of their inhibition American Journal of Clinical Oncology 39 1 98–106
[15]. Iranzo P Callejo A Assaf J D Molina G Lopez D E Garcia-Illescas D & Falgàs N 2022 Overview of checkpoint inhibitors mechanism of action: Role of immune-related adverse events and their treatment on progression of underlying cancer Frontiers in Medicine 9 875974
[16]. Sivanandam V LaRocca C J Chen N G Fong Y & Warner S G 2019 Oncolytic viruses and immune checkpoint inhibition: The best of both worlds Molecular Therapy Oncolytics 13 93–106
[17]. Klöppel G 2017 Neuroendocrine neoplasms: Dichotomy origin and classifications Visceral Medicine 33 5 324–330
[18]. Mehnert J M Bergsland E O’Neil B H Santoro A Schellens J H M Cohen R B & Gupta S 2020 Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study Cancer 126 13 3021–3030
[19]. Li Y L Cheng Z X Yu F H Tian C & Tan H Y 2022 Advances in medical treatment for pancreatic neuroendocrine neoplasms World Journal of Gastroenterology 28 20 2163–2175
[20]. Goodman A 2019 Dual-immunotherapy approach shows efficacy in high-grade neuroendocrine tumors ASCO Post Retrieved August 19 2024 from: https://ascopostcom/issues/may-10-2019/dual-immunotherapy-in-high-grade-neuroendocrine-tumors/
[21]. Pan W X Zhang X M Hao S L & Han W 2023 Progress in immunotherapy for neuroendocrine neoplasm of the digestive system World Journal of Gastroenterology 29 26 4174–4185
[22]. Egal E S A Jacenik D Soares H P & Beswick E J 2021 Translational challenges in pancreatic neuroendocrine tumor immunotherapy Biochimica et Biophysica Acta BBA - Reviews on Cancer 1876 2 188640
[23]. Kiyotani K Toyoshima Y & Nakamura Y 2021 Personalized immunotherapy in cancer precision medicine Cancer Biology & Medicine 18 4 955–965
Cite this article
Lau,C.C. (2024). The advancement in the application of immune checkpoint inhibitors in pNET. Theoretical and Natural Science,73,1-7.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. American Cancer Society nd What is a pancreatic neuroendocrine tumor? Retrieved August 7 2024 from: https://www.cancer.org/cancer/types/pancreatic-neuroendocrine-tumor/about/what-is-pnet.html
[2]. Gubbi S Vijayvergia N Yu J Q Klubo-Gwiezdzinska J & Koch C A 2022 Immune checkpoint inhibitor therapy in neuroendocrine tumors Hormone and Metabolic Research 54 12 795
[3]. Panda A Garg I Johnson G B Truty M J Halfdanarson T R & Goenka A H 2019 Molecular radionuclide imaging of pancreatic neoplasms The Lancet Gastroenterology & Hepatology 4 7 559–570
[4]. Kendi A T Halfdanarson T R Packard A Dundar A & Subramaniam R M 2019 Therapy with 177Lu-DOTATATE: Clinical implementation and impact on care of patients with neuroendocrine tumors American Journal of Roentgenology 213 2 309–317
[5]. Hopper A D Jalal M & Munir A 2019 Recent advances in the diagnosis and management of pancreatic neuroendocrine tumours Frontline Gastroenterology 10 3 269–274
[6]. Pennock G K & Chow L Q M 2015 The evolving role of immune checkpoint inhibitors in cancer treatment The Oncologist 20 7 812–822
[7]. Zhang H Dai Z Wu W Wang Z Zhang N Zhang L & He X 2021 Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer Journal of Experimental & Clinical Cancer Research 40 1 184
[8]. He X & Xu C 2020 Immune checkpoint signaling and cancer immunotherapy Cell Research 30 8 660–669
[9]. Gomatou G Tzilas V Kotteas E Syrigos K & Bouros D 2020 Immune checkpoint inhibitor-related pneumonitis Respiration 99 11 932–942
[10]. Nature Communications 2020 A decade of immune-checkpoint inhibitors in cancer therapy Nature Communications
[11]. Han Y Liu D & Li L 2020 PD-1/PD-L1 pathway: Current researches in cancer American Journal of Cancer Research 10 3 727–742
[12]. Jiang Y Chen M Nie H & Yuan Y 2019 PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations Human Vaccines & Immunotherapeutics 15 5 1111–1122
[13]. Immune Checkpoint Inhibitors - NCI. 24 Sept. 2019, https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors. nciglobal, ncienterprise.
[14]. Buchbinder E I & Desai A 2016 CTLA-4 and PD-1 pathways: Similarities differences and implications of their inhibition American Journal of Clinical Oncology 39 1 98–106
[15]. Iranzo P Callejo A Assaf J D Molina G Lopez D E Garcia-Illescas D & Falgàs N 2022 Overview of checkpoint inhibitors mechanism of action: Role of immune-related adverse events and their treatment on progression of underlying cancer Frontiers in Medicine 9 875974
[16]. Sivanandam V LaRocca C J Chen N G Fong Y & Warner S G 2019 Oncolytic viruses and immune checkpoint inhibition: The best of both worlds Molecular Therapy Oncolytics 13 93–106
[17]. Klöppel G 2017 Neuroendocrine neoplasms: Dichotomy origin and classifications Visceral Medicine 33 5 324–330
[18]. Mehnert J M Bergsland E O’Neil B H Santoro A Schellens J H M Cohen R B & Gupta S 2020 Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study Cancer 126 13 3021–3030
[19]. Li Y L Cheng Z X Yu F H Tian C & Tan H Y 2022 Advances in medical treatment for pancreatic neuroendocrine neoplasms World Journal of Gastroenterology 28 20 2163–2175
[20]. Goodman A 2019 Dual-immunotherapy approach shows efficacy in high-grade neuroendocrine tumors ASCO Post Retrieved August 19 2024 from: https://ascopostcom/issues/may-10-2019/dual-immunotherapy-in-high-grade-neuroendocrine-tumors/
[21]. Pan W X Zhang X M Hao S L & Han W 2023 Progress in immunotherapy for neuroendocrine neoplasm of the digestive system World Journal of Gastroenterology 29 26 4174–4185
[22]. Egal E S A Jacenik D Soares H P & Beswick E J 2021 Translational challenges in pancreatic neuroendocrine tumor immunotherapy Biochimica et Biophysica Acta BBA - Reviews on Cancer 1876 2 188640
[23]. Kiyotani K Toyoshima Y & Nakamura Y 2021 Personalized immunotherapy in cancer precision medicine Cancer Biology & Medicine 18 4 955–965









