1. Introduction
Cancer remains a significant global health challenge, with approximately 20 million new cases and 9.7 million deaths reported in 2022. The five-year survival rate for cancer patients stood at around 53.5 million. Lung, breast, and colorectal cancers were among the most prevalent types [1, 2] (Figure 1). Notably, breast cancer alone affected 2.3 million women worldwide in 2022, resulting in 670,000 deaths [3].
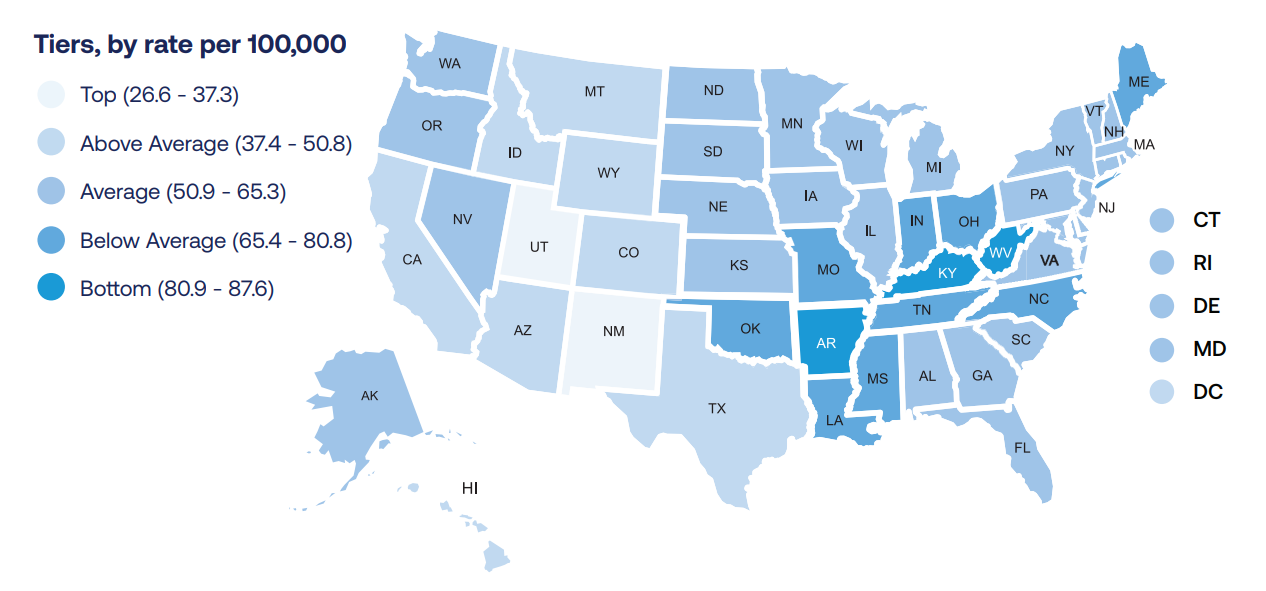
Figure 1. New cases in America of lung cancer in 2022.
Traditional cancer treatments, while often life-saving, come with considerable limitations. The conventional approach of targeting tumors for eradication can overlook the underlying causes and risk factors of cancer. This "one-size-fits-all" strategy may prove ineffective in addressing the specific nature of the disease and preventing its recurrence. Moreover, some treatment methods may inadvertently compromise the immune system, the body's natural defense mechanism against cancer cells. This can hinder the body's ability to combat cancer effectively and increase susceptibility to infections and other health complications. Chemotherapy and radiotherapy, despite their crucial role in cancer treatment, often lead to severe side effects such as nausea, vomiting, fatigue, and long-term bone marrow suppression, significantly impacting patients' quality of life during and after treatment [4].
In light of these challenges, mRNA cancer vaccines have emerged as a promising alternative, offering several advantages over traditional treatments. These vaccines can be tailored to target specific antigens found on cancer cells, providing a more personalized and potentially more effective treatment approach with fewer side effects. By stimulating a targeted immune response against cancer cells, mRNA vaccines harness the power of the body's immune system to fight cancer more effectively.
The development of mRNA cancer vaccines is rooted in the biological basis of mRNA synthesis and expression. These vaccines are designed to work against existing cancer cells by targeting tumor-associated antigens that are either absent or present at lower levels in normal cells. By introducing these antigens, the vaccines help the immune system recognize and react to cancer cells, ultimately destroying them.
Various delivery systems have been developed to enhance the efficacy of mRNA vaccines. These include naked mRNA injection, electroporation for dendritic cell-based vaccines, peptide-based delivery using protamine, polymer-based delivery, lipid-based nanoparticles, cationic nano-emulsions, and virus-like replicon particles (VRPs). Each of these systems offers unique advantages in terms of mRNA protection, cellular uptake, and antigen expression [5].
The rapid development and production capabilities of mRNA vaccines, coupled with their potential for combination therapy and scalability, make them a promising avenue for cancer treatment. As research in this field progresses, mRNA cancer vaccines have the potential to revolutionize cancer therapy by offering a more targeted, personalized, and potentially more effective approach to fighting cancer.
2. Current achievements
mRNA cancer vaccines have already shown great promise in clinical trials, with multiple vaccines currently in use or under development [6]. The field has made significant strides in recent years, demonstrating the potential of this innovative approach to cancer treatment. Some of the current achievements include:
Successful Development and Approval of mRNA Vaccines: The rapid development and approval of mRNA vaccines for COVID-19 have paved the way for further advancements in mRNA technology. This success has not only validated the mRNA platform but also accelerated research and development in the field of cancer vaccines.
Positive Clinical Trial Results: Early-phase clinical trials of mRNA cancer vaccines have shown encouraging results across various types of cancer. Notably, trials targeting melanoma and breast cancer have demonstrated the potential of these vaccines to stimulate effective immune responses against cancer cells.
Personalized Cancer Treatments: mRNA technology offers the unique ability to create personalized cancer treatments. By targeting specific tumor antigens or mutations unique to an individual's cancer, these vaccines can potentially provide highly tailored and effective therapies.
Strong Immune Response Stimulation: mRNA cancer vaccines have shown the ability to stimulate a robust immune response against cancer cells. This heightened immune activation could potentially lead to improved treatment outcomes, offering hope for patients with difficult-to-treat cancers.
These achievements represent significant progress in the field of cancer immunotherapy and highlight the potential of mRNA cancer vaccines to transform cancer treatment paradigms. As research continues and more clinical data becomes available, the full impact of these innovations on cancer care will become increasingly clear.
3. Safety and tolerability
The safety and tolerability of mRNA cancer vaccines have been extensively studied in clinical trials, with results indicating that these vaccines are generally well-tolerated and safe for patients. This chapter explores the key aspects of their safety profile, including common side effects, potential concerns, and long-term safety considerations.
Clinical trials have revealed that the most common side effects of mRNA vaccines are mild to moderate local reactions at the injection site, such as pain, redness, or swelling. These reactions are typically temporary and resolve on their own without intervention. In addition to local reactions, some patients may experience systemic effects following vaccination, including fatigue, headache, muscle aches, and fever. These systemic reactions are usually mild to moderate in severity and tend to subside within a few days.
While the overall safety profile of mRNA vaccines is favorable, there are some potential concerns that warrant attention. Severe allergic reactions, although rare, can occur with mRNA vaccines. Healthcare providers are trained to manage and treat any allergic reactions that may arise. Patients with a history of severe allergic reactions to components of the vaccine are advised to consult with their healthcare provider before receiving an mRNA vaccine. There have also been concerns about potential autoimmune reactions triggered by mRNA vaccines. However, current clinical data have not shown a significant increase in autoimmune diseases among vaccinated individuals.
The long-term safety of mRNA vaccines is an area of ongoing research, as these vaccines are relatively new in the field of cancer treatment. While long-term safety data are still being collected and monitored, early studies and real-world evidence suggest that mRNA vaccines have a favorable safety profile. Continuous monitoring and reporting of adverse events remain crucial as these vaccines become more widely used in cancer treatment.
Special considerations should be given to certain populations, such as pregnant and breastfeeding women. These individuals should discuss the potential risks and benefits of mRNA vaccination with their healthcare providers to make informed decisions. Healthcare providers play a critical role in conducting individual risk assessments for each patient, ensuring that the benefits of vaccination outweigh any potential risks.
Overall, the safety and tolerability profile of mRNA cancer vaccines appears to be favorable, with the majority of reported side effects being mild to moderate and transient. It is essential for healthcare providers to carefully monitor patients receiving mRNA vaccines, promptly address any adverse events that may arise, and provide comprehensive information to patients about potential side effects and their management.
As research in this field progresses and more data become available, our understanding of the long-term safety profile of mRNA cancer vaccines will continue to evolve. This ongoing research may lead to further refinements in vaccine design and administration protocols, potentially resulting in even safer and more effective cancer treatments. The promising safety profile of mRNA vaccines, combined with their potential therapeutic benefits, positions them as a valuable tool in the growing arsenal of cancer immunotherapies.
4. Current challenges
Despite the promising advancements in mRNA cancer vaccines, several challenges remain that require further research and development. This chapter explores the key obstacles faced in the field, focusing on the diversity of immune responses, stability and delivery efficiency of mRNA, and limitations on efficacy in advanced cancer.
4.1. Diversity of immune responses
The body's initial line of defense against microbial infections is the innate immune system. For the purpose of identifying molecular signatures like pathogen-associated molecular patterns (PAMPs), it is dependent on germline-encoded receptors called pattern recognition receptors (PRRs). Furthermore, innate host defense and inflammatory reactions can be triggered by molecular patterns called damage-associated molecular patterns (DAMPs) that are expressed on injured host cells.
Enhancing immunotherapy requires bridging the gap between innate and adaptive immunity. PAMPs or DAMPs bind to PRRs in response to an aberrant signal—such as an infection or cancer—triggering an antigen-presenting cell (APC) response. When activated, APCs release cytokines and increase the production of co-stimulatory molecules, both of which are crucial for initiating, intensifying, and triggering adaptive immune responses. CD8+ cytotoxic lymphocytes recognize diseased or distressed cells via MHC class I molecules, and they then release cytotoxic proteins to cause apoptosis [7].
4.2. Stability and delivery efficiency of mRNA
The process of producing mRNA itself is quite simple, but getting an mRNA therapy into a cell at the right dose is a very difficult task. The most important is steadiness. Nuclease enzymes usually break down mRNA in a matter of minutes. To combat this, numerous scientists have discovered ways to modify specific chemical characteristics of the mRNA transcript, thereby decreasing its attractiveness to nucleases.
It would be necessary to individually tune every new mRNA for a specific therapeutic use before producing and formulating it for administration using a largely standard process. mRNA has the potential to serve as a broadly applicable platform for expedited medication and vaccine development. For the complete process of mRNA synthesis, optimization, and distribution, this procedure needs essential equipment and materials [8].
4.3. Limitations on efficacy in advanced cancer
The effectiveness of mRNA vaccines in advanced cancer is restricted by a number of issues. It is still challenging to determine whether HLA alleles have tumor-specific mutations or non-conforming sequences and to anticipate the relevant neoepitopes. Future challenges that will need to be overcome include the technological and legal barriers that will result from the requirement for quick and extensive good manufacturing practice production of personalized mRNA vaccines. Furthermore, it's critical to validate the most practical vaccination administration techniques. The mode of administration affects vaccine effectiveness and impacts the distribution of mRNA. Regional antigen-presenting cells may readily process mRNA given subcutaneously and intradermally, however these administration methods frequently result in significant local injection-site responses.
In conclusion, while mRNA cancer vaccines show great promise, overcoming these challenges is essential for their widespread clinical application. Continued research into immune response mechanisms, mRNA stability and delivery methods, and strategies to enhance efficacy in advanced cancer stages will be crucial for advancing this innovative approach to cancer treatment.
5. Future directions of development
As mRNA cancer vaccines continue to evolve, researchers are exploring various strategies to enhance their efficacy and overcome current limitations. This chapter discusses the future directions of mRNA vaccine development, with a focus on combining these vaccines with other immunotherapies.
5.1. The use of mRNA vaccines in combination with other immunotherapies
While mRNA therapeutic cancer vaccines offer numerous benefits, they also face several limitations. The rapid degradation of naked mRNA by RNAses in the extracellular environment and inefficient internalization by antigen-presenting cells pose significant challenges. Additionally, mRNA's natural property of activating downstream interferon-related pathways can evoke the innate immune system. Although this response enhances overall immune activation, it can also promote mRNA degradation, counteracting antigen expression. Furthermore, the presence of double-stranded RNAs as impurities can strengthen the innate immune response, potentially limiting mRNA translation [9].
In order to overcome these drawbacks and improve the effectiveness of mRNA vaccines, scientists are investigating combination therapies with alternative immunotherapeutic strategies. Combining chemotherapy and mRNA vaccines is one interesting approach. Chemotherapy can directly impact tumor cells and, by modulating the immune system, increase therapeutic efficacy. According to Marij et al., mice's survival rates were increased when an SLP therapeutic cancer vaccination was combined with paclitaxel and carboplatin. Chemotherapy-induced alterations in the makeup of the bone marrow cell population were blamed for this improvement. These results were further supported by a clinical experiment (NCT02128126), in which the HPV16 SLP vaccine ISA101 in conjunction with chemotherapy caused an increase in T cell proliferation in response to recall antigens in addition to depleting aberrant myeloid cells. Patients with HPV16-positive advanced stage cervical cancer experienced a robust and long-lasting antigen-specific T cell response as a result, which was linked to better clinical outcomes. These results show that chemotherapy can alter immune responses and improve the effectiveness of cancer vaccines.
Another promising approach involves combining mRNA vaccines with immune checkpoint inhibitors (ICIs). ICIs have showed promise as cancer therapeutic techniques. However, their effectiveness remains restricted, as only a few individuals benefit from ICI therapy due to poor pre-existing cytotoxic T cell responses. Cancer vaccines can stimulate T cells to convert the tumor immune microenvironment from "cold" to "hot." Combining ICIs with cancer vaccines is an exciting technique for improving immune treatment responses, warranting more research in future clinical studies. For instance, a tailored SLP cancer vaccine, NEO-PV-01, led to substantial pathological responses in 9 individuals with melanoma who had limited or only partial responses to nivolumab. Moreover, NEO-PV-01 plus nivolumab caused epitope spread, resulting to the generation of neoantigens that supplied novel targets for T cells. NEO-PV-01 combined with anti-PD-1 and chemotherapy may become the standard first-line treatment for non-squamous NSCLC if more clinical research is done.
These combination approaches highlight the potential for synergistic effects between mRNA vaccines and other immunotherapies, paving the way for more effective cancer treatments in the future. As research in this field progresses, it is likely that more innovative combinations and strategies to optimize the efficacy of mRNA cancer vaccines will be developed.
5.2. New technological advancements and innovations
mRNA cancer vaccines represent a cutting-edge innovation in cancer treatment, offering a highly personalized and targeted approach. These vaccines function by delivering mRNA sequences that encode tumor-specific antigens, which prompt an immune response aimed at identifying and destroying cancer cells. One of the most significant advancements in mRNA cancer vaccines is their ability to be rapidly customized to target the unique mutations present in an individual's cancer. This tailored approach offers the potential for more precise targeting of cancer cells, which may result in improved treatment outcomes and fewer side effects compared to conventional therapies such as chemotherapy.
Moreover, mRNA vaccines have demonstrated the capacity to elicit a durable immune response, which could provide long-lasting protection. This characteristic is particularly advantageous for preventing cancer recurrence or the spread of metastatic disease. The field of mRNA vaccine technology is advancing rapidly, with ongoing research efforts focusing on optimizing delivery systems, enhancing vaccine stability, and improving immune activation. These innovations may further increase the efficacy and efficiency of cancer vaccines in the future.
Overall, mRNA cancer vaccines are poised to become a transformative element in cancer immunotherapy, offering new hope to patients and potentially reshaping how cancer is treated.
5.3. The potential of prophylactic mRNA vaccines
Prophylactic mRNA vaccines hold tremendous promise in the prevention of infectious diseases, including those caused by viruses such as influenza, Zika, and COVID-19. These vaccines work by delivering mRNA that encodes specific antigens, triggering the immune system to mount a defense without causing the disease. One of the key advantages of mRNA vaccines is their ability to be developed and manufactured swiftly. Unlike traditional vaccine platforms, mRNA vaccines can be designed and produced rapidly in response to emerging infectious disease outbreaks, facilitating a more timely and effective public health response.
Additionally, mRNA vaccines have shown the ability to induce a strong and sustained immune response, potentially offering longer-lasting protection against infections. This could be particularly beneficial for diseases that often require frequent booster vaccinations, as mRNA vaccines might provide durable immunity with fewer doses. Furthermore, the flexibility of mRNA vaccine technology allows for quick adjustments to address new strains or mutations of viruses, making them highly adaptable to evolving pathogens.
In summary, prophylactic mRNA vaccines have the potential to revolutionize infectious disease prevention by providing a rapid, effective, and adaptable method of protecting populations against a wide range of pathogens. Continued research and innovation in this area may significantly enhance global health outcomes and pandemic preparedness.
6. Conclusion
mRNA cancer vaccines represent a significant advancement in personalized cancer immunotherapy. Clinical trials have demonstrated their potential in terms of safety, tolerability, and efficacy. However, challenges remain, including the diversity of immune responses, mRNA stability and delivery efficiency, and limitations in advanced cancer stages.
The future of mRNA cancer vaccines lies in addressing these challenges through continued research and innovation. Combination therapies, particularly with chemotherapy and immune checkpoint inhibitors, show promise in enhancing treatment efficacy. As technology advances, improvements in mRNA design, delivery systems, and manufacturing processes are expected to further optimize these vaccines.
While obstacles persist, the potential of mRNA cancer vaccines to revolutionize cancer treatment is substantial. Their ability to provide personalized, targeted therapy offers new hope in the fight against cancer. Ongoing research and clinical trials will be crucial in realizing the full potential of this innovative approach, potentially transforming the landscape of cancer therapy in the years to come.
References
[1]. World Health Organization. (2024, February 1). Global Cancer Burden growing, Amidst Mounting Need for Services. Www.who.int. https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services
[2]. State Data. (2022). Www.lung.org. https://www.lung.org/research/state-of-lung-cancer/states
[3]. World. (2024, March 13). Breast cancer. Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=In
[4]. Verhoef, M. J., Rose, M. S., White, M., & Balneaves, L. G. (2008). Declining conventional cancer treatment and using complementary and alternative medicine: a problem or a challenge?. Current oncology (Toronto, Ont.), 15 Suppl 2(Suppl 2), s101–s106. https://doi.org/10.3747/co.v15i0.281
[5]. Nitika, Wei, J., & Hui, A. M. (2022). The Delivery of mRNA Vaccines for Therapeutics. Life (Basel, Switzerland), 12(8), 1254. https://doi.org/10.3390/life12081254
[6]. Cao, Q., Fang, H., & Tian, H. (2024). mRNA vaccines contribute to innate and adaptive immunity to enhance immune response in vivo. Biomaterials, 310, 122628. https://doi.org/10.1016/j.biomaterials.2024.122628
[7]. Zhai, J., Cote, T., & Chen, Y. (2024). Challenges and advances of the stability of mRNA delivery therapeutics. Nucleic acid insights, 1(2), 101–113. https://doi.org/10.18609/nai.2024.015
[8]. Miao, L., Zhang, Y., & Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Molecular cancer, 20(1), 41. https://doi.org/10.1186/s12943-021-01335-5
[9]. Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines - a new era in vaccinology. Nature reviews. Drug discovery, 17(4), 261–279. https://doi.org/10.1038/nrd.2017.243
Cite this article
Wei,Z. (2024). mRNA cancer vaccines: revolutionizing personalized immunotherapy: progress, challenges, and future directions. Theoretical and Natural Science,74,86-92.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. World Health Organization. (2024, February 1). Global Cancer Burden growing, Amidst Mounting Need for Services. Www.who.int. https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services
[2]. State Data. (2022). Www.lung.org. https://www.lung.org/research/state-of-lung-cancer/states
[3]. World. (2024, March 13). Breast cancer. Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/breast-cancer#:~:text=In
[4]. Verhoef, M. J., Rose, M. S., White, M., & Balneaves, L. G. (2008). Declining conventional cancer treatment and using complementary and alternative medicine: a problem or a challenge?. Current oncology (Toronto, Ont.), 15 Suppl 2(Suppl 2), s101–s106. https://doi.org/10.3747/co.v15i0.281
[5]. Nitika, Wei, J., & Hui, A. M. (2022). The Delivery of mRNA Vaccines for Therapeutics. Life (Basel, Switzerland), 12(8), 1254. https://doi.org/10.3390/life12081254
[6]. Cao, Q., Fang, H., & Tian, H. (2024). mRNA vaccines contribute to innate and adaptive immunity to enhance immune response in vivo. Biomaterials, 310, 122628. https://doi.org/10.1016/j.biomaterials.2024.122628
[7]. Zhai, J., Cote, T., & Chen, Y. (2024). Challenges and advances of the stability of mRNA delivery therapeutics. Nucleic acid insights, 1(2), 101–113. https://doi.org/10.18609/nai.2024.015
[8]. Miao, L., Zhang, Y., & Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Molecular cancer, 20(1), 41. https://doi.org/10.1186/s12943-021-01335-5
[9]. Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines - a new era in vaccinology. Nature reviews. Drug discovery, 17(4), 261–279. https://doi.org/10.1038/nrd.2017.243









