1. Introduction
About 20 million new cases of cancer were detected in 2022, and 9.7 million people died from it globally, according to the International Agency for Research on Cancer's most recent estimate of the incidence and mortality of 36 forms of cancer in 185 countries. According to this report, lung cancer had the highest incidence rate globally in 2022, accounting for nearly 2.5 million cases, followed by breast cancer [1]. These data warn us that cancer is a major public health issue that poses a threat to human health and social stability. The widely used treatment methods for cancer are chemotherapy and radiotherapy. Although these traditional treatment methods can inhibit the growth, metastasis, and deterioration of tumor cells, chemotherapy drugs have poor targeting and significant toxic side effects on the human body [2]. While inhibiting tumor cells, chemotherapy and radiotherapy drugs often damage the proliferation of normal cells, including hair follicle cells and bone marrow stem cells. This has the potential to cause severe side effects and adverse reactions, such as postoperative depression in patients.
In order to reduce these side effects, natural medicines have been widely favored in the development of anti-tumor drugs in recent years. Notable biological properties of natural medicines include antioxidant, antiviral, and anticancer actions. They also have the benefits of low toxicity, abundant resources, and easy accessibility [3]. The natural active ingredient quercetin is widely found in fruits, plants, vegetables, and traditional Chinese medicine. It is easy to obtain and more affordable compared to chemotherapy drugs. Moreover, as a high and abundant flavonoid compound, quercetin can inhibit the development of various cancers, directly promote apoptosis in tumor cells [4], and have minimal toxic side effects on normal cells. Therefore, it has garnered significant attention from medical researchers. This paper examines quercetin's anti-cancer potential and mode of action while summarizing its current advancements in cancer treatment.
2. Chemical Structure and Biological Properties of Quercetin
3,3', 4', 5,7-pentahydroxyflavone is the scientific name for quercetin, and its molecular formula is C15H1007. It consists of two benzene rings and one pyran ring (Figure 1) [5]. The hydroxyl and double bonds connected by the benzene ring in the structure play a crucial role in quercetin's antioxidant activity [6]. The strength of its antioxidant ability is influenced by the substituents on the B and C rings [7, 8]. Quercetin is a low-molecular-weight flavonoid compound, classified among the lipophilic compounds. It exhibits low solubility in water, poor water solubility, and low bioavailability. Quercetin has attracted much attention for its excellent anti-cancer and antioxidant functions, as well as its role in inhibiting inflammation and regulating the immune system. However, its extremely low water solubility and insufficient human utilization constitute an important obstacle in the field of clinical treatment and scientific research [9]. Therefore, researchers have explored various solutions to improve the bioavailability of quercetin, including developing new dosage forms, solid dispersants, and emulsifiers. Techniques such as embedding technology, polymer packaging materials like nanoparticles and micelles, or generating complexes have been used to enhance quercetin absorption and increase its concentration in human plasma [10, 11]. Quercetin mainly exists in the form of glycosides in nature. The different properties of sugar groups connecting the structural skeleton of quercetin can influence its water solubility and absorption in the human body [6]. Quercetin glycosides in their conjugated form are more easily absorbed by the body than free quercetin [12].
Quercetin, as a natural component with significant anti-tumor properties, has shown excellent application prospects for the prevention and treatment of tumors. At the right dose, the substance has no adverse effect on the growth and reproduction of normal cells but can fight against tumor cells through a variety of interaction mechanisms. A number of studies have confirmed the effectiveness of quercetin in a variety of tumor model systems, which can not only stimulate the programmed death of tumor cells, but also block their growth and spread paths. [12, 13]. The capacity of quercetin to function as an antioxidant at low doses mostly indicates its preventative effect on tumors; yet, at high concentrations, quercetin transforms into a pro-oxidant, thereby exerting a chemotherapeutic effect on tumors [13, 14]. Furthermore, human clinical trials have reported no toxic side effects associated with quercetin [15].
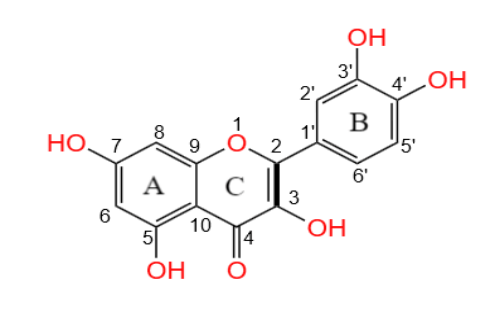
Figure 1. The chemical structure of Quercetin
3. Mechanisms of Quercetin in Anti-Cancer Activity
Quercetin, as the most abundant flavonoid, has significant antioxidant effects and effectively clears free radicals in the body. Through their effects on cell survival, proliferation, and death, reactive oxygen species (ROS) can cause carcinogenesis. Compared to healthy cells, the concentration of reactive oxygen species in cancer cells showed an upward trend; this enables cancer cells to survive in a high level of reactive oxygen species (ROS) environment while maintaining cellular activity and functional integrity, avoiding cell damage or death caused by oxidative stress. Quercetin can be used in targeted cancer therapy, as the high ROS levels in cancer cells make them particularly vulnerable to quercetin’s effects, ultimately promoting cell apoptosis [16]. Quercetin can induce cell apoptosis through ROS-mediated signaling pathways, including the p38/ASK1/AMPKα1/COX2 axis [17]. Additionally, quercetin promotes autophagy and triggers death in cancer cells via a number of mechanisms: it activates caspase-3, By regulating core signaling pathways such as AKT and mTOR, the expression of β-catenin is reduced, and the stability of hypoxia-inducing factor (HIF-α) is maintained [18].
Quercetin causes cell cycle arrest, which stops cancer cells from proliferating, effectively preventing their progression at critical stages of the cell cycle. One study revealed that by treating HeLa cells with quercetin, it was observed that there was a significant stagnation of the cell cycle in the G2 and M phases, leading to the accumulation of intracellular ROS levels and triggering the release and activation of mitochondrial cytochrome C [19]. This process affects the apoptotic pathway and ultimately induces tumor cell death. Another study found that quercetin can cause cell apoptosis by inhibiting the growth and migration of several multiple myeloma cell lines and blocking the G1-M phase of the cell cycle [20]. Moreover, studies on the melanoma cell line A375 [21] revealed that β-catenin, DVL2, cyclin D1, and Axin2 proteins play a key role in quercetin's regulation of Wnt/β-catenin signaling pathways, which interact with each other to jointly inhibit cell proliferation.
Meanwhile, the occurrence and development of many tumors are closely related to abnormal angiogenesis, and the imbalance of this process is directly related to the expansion and diffusion of tumor cells. In fact, dysplasia of angiogenesis is considered to be a decisive factor in maintaining the survival of otherwise normal cells in malignant tumors [22]. In addition, metastasis of cancer cells is essentially their escape from the surveillance of the immune system, split off from the primary tumor, and then enter nearby healthy cells [23]. Quercetin reduces the possibility of cancer cell growth and metastasis by reducing vascular development at the source. It can also inhibit the expression of AKT protein in malignant tumors of the human reproductive system by targeting the angiogenesis pathway transmitted by VEGFR-2. Quercetin has a significant effect on slowing tumor growth [18], which plays an indispensable role in curbing the progression of malignant tumors by intervening in many cell signal transmission pathways and controlling angiogenesis.
4. Pre-Clinical Evidence and Clinical Studies of Quercetin in Cancer Therapy
A study found that quercetin alone has significant cytotoxic effects on PC3 prostate cancer cells and CD44+/CD133+ stem cells. Using quercetin and MKsiRNA together was more effective at killing cells and stopping their growth in the G1 phase of the cell cycle than using quercetin alone. By knocking down the MK gene, quercetin can significantly inhibit the migration of CD44+/CD133+ stem cells. Further studies showed that this combined treatment reduced the levels of proteins required for the migration and reproduction of prostate cancer cells while inhibiting the activation of PI3K, AKT, and ERK1/2. It is speculated that the MK gene may enhance the inhibitory effect of quercetin on the survival and metastasis of prostate cancer stem cells by regulating the PI3K/AKT and MAPK/ERK signaling pathways. This result suggests that using quercetin to treat tumor stem cells may be an important research direction for its recurrence, metastasis, and drug resistance [24]. Quercetin activates a series of proteases in the intracellular apoptotic pathway, including caspase-3, -8, and -9. They regulate the process of apoptosis by promoting the function of Bax and Bad proteins and inhibiting the activity of anti-apoptotic proteins such as Bcl XL, Bcl-2, and Mcl-1.
Simultaneously, quercetin stimulates mitochondria to release cytochrome C into the cytoplasm [12], thereby inducing cell apoptosis. Numerous investigations have verified that quercetin's anti-tumor impact is demonstrated by preventing the growth of cancer cells by altering the transmission of cell information and lowering the production of proteins linked to cell growth, enhancing cancer cell death signals, and promoting autophagy. MCF-7 breast cancer cell lines were cultured for 24, 48, and 72 hours in vitro with quercetin nanoparticles at concentrations ranging from 1 to 100 micromoles. The experimental data revealed that in this concentration range, quercetin nanoparticles can effectively weaken the survival ability of MCF-7 cells, slow down their growth rate, and significantly inhibit the formation of cell colonies [25]. Lee et al. and Van the Woude et al. found that quercetin-induced reactive oxygen species (ROS) could activate AMPK in the MCF-7 cell line [26, 27].
Further analysis showed that the effect of quercetin on apoptosis and proliferation was closely related to the concentration of quercetin used. High concentrations of quercetin (maximum tested dose of 100 μM) reduced cell survival rate, but concentrations ranging from 10 to 70 μM showed a significant enhancement effect on cell growth and proliferation. Xu et al. [28] observed that quercetin extract with concentrations as low as 5-20 μM can promote cell proliferation when used to treat MCF-7 cells, increasing estrogen receptor (ER) membrane protein levels, and showed that the number of MDA-MB-231 cells increased slightly under a higher concentration of 100 μM quercetin. However, in contrast, this concentration of quercetin induced cell cycle arrest and apoptosis while showing inhibitory effects on proliferation in both cell lines.
Quercetin has antioxidant and free radical scavenging properties, making it an effective chemopreventive natural medicine and an ideal treatment for cancer prevention. Research has shown that patients who consume large amounts of flavonoid-rich foods can reduce their risk of cancer [29]. A study has shown that regularly administering broccoli sprouts rich in quercetin active ingredients to patients is beneficial for pancreatic ductal adenocarcinoma (PDA) [30]. According to a different study, by causing oxidative stress, patients who take 500 mg of quercetin every day for six months in a row can prevent prostate cancer.
Quercetin has a wide range of pharmacological effects, but its low bioavailability and hydrophobicity limit its use in anti-cancer therapy. Quercetin is mainly taken up by the digestive tract in the gut in the form of glycosides. However, affected by the instability of the gastrointestinal environment, the substance is prone to first-pass metabolism, resulting in its absorption efficiency in the human body being not satisfactory. In addition, quercetin may cause adverse reactions to organs such as the liver and kidney when the dose is increased, while low-dose quercetin treatment may not be effective. Therefore, a precise dosage is required for people to take quercetin [29]. However, current research has shown [31, 32] that the gut microbiota can produce glycosidases and other molecules that help quercetin transfer to smaller, more easily absorbed molecules in the human body. Meanwhile, quercetin can increase its bioavailability by forming ion complexes such as glucan quercetin complexes and metal nanoparticles for delivery [33].
5. Potential for Quercetin in Combination Cancer Therapy
The combined use of quercetin and various chemical small molecule drugs highlights its ability to regulate signaling pathways and block cell cycle. In this way, the dosage of anti-tumor drugs can be reduced while improving the overall effectiveness and safety of the drugs [34]. Quercetin has great research prospects as an adjuvant therapy for cancer, and resistance to gemcitabine (GEM) is a challenge faced by clinical doctors and advanced cancer patients. Liu and colleagues explored in detail the effect of quercetin on gem-resistant cancer cells and its mechanism of action [35]. The study found that quercetin showed a significant inhibitory effect on gem-resistant cancer cell lines, not only effectively slowing down their growth rate but also significantly increasing the rate of apoptosis.
Compared to using GEM alone, the combination therapy of quercetin and GEM significantly enhances anti-cancer efficacy. Tumor stem cells exhibit drug and radiation resistance; however, the oxidative stress capabilities of quercetin can inhibit ataxia telangiectasia mutated protein (ATM), promoting tumor radiosensitization [36]. Studies have also revealed that quercetin can effectively block the transfer of Y-Box-binding protein-1 to the nucleus by reducing the expression of P-glycoprotein [37]. This significantly improves the lethality of doxorubicin, paclitaxel, and vincristine against multi-drug-resistant breast cancer cells and breast cancer stem cells. At the same time, quercetin combined with chemotherapy and radiotherapy can enhance the body's sensitivity to drugs and play a protective role in normal cells, greatly reducing the degree of toxic side effects. In the study of the AGS human gastric cancer cell line, the effects of combined treatment with quercetin and SN-38 (an active metabolite of irinotican and a DNA topoisomerase inhibitor) on cell viability, apoptosis rate, and β-catenin expression level were similar to those of high-intensity SN-38 alone. Further comparison showed that, compared with irinotecan alone, combined quercetin treatment was more effective in inhibiting tumor growth [38].
6. Summary
Plant-derived natural medicines have great potential to replace anti-tumor drugs. In a number of animal experiments and in vitro studies, quercetin has shown excellent antioxidant properties, and its anti-cancer effect is significant. The compound exerts its anticancer effects through a variety of mechanisms, such as stimulating programmed cell death, regulating cell cycle progression, blocking angiogenesis, and intervention of key signaling pathways such as PI3K, Wnt/β-catenin, and p35. Quercetin, as an effective chemopreventive compound, has the advantages of low toxicity, wide pharmacological effects, enhanced radiotherapy efficacy, abundant resources, and easy accessibility. It inhibits cancer cells proliferation without affecting the normal growth of healthy cells. Its synergistic effects with chemotherapy and radiotherapy can improve treatment sensitivity, positioning it as an ideal candidate for cancer chemotherapy and as an adjunct therapy. Although quercetin has a variety of pharmacological activities, its clinical application is greatly restricted by its poor water solubility, low bioavailability, and susceptibility to first-pass effects. Currently, these drawbacks can be addressed by using biopolymers and nanoparticles as carriers to enhance the delivery of quercetin or by formulating it into ion complexes to increase bioavailability. There is substantial preclinical evidence supporting the pharmacological effects of quercetin, and ongoing clinical trials are further confirming its anticancer potential. However, the widespread use of quercetin in clinical practice has been inhibited by the limited and insufficient scope of current clinical trial data. To establish the therapeutic efficacy of quercetin against tumors, a comprehensive and sufficient number of clinical trials are essential.
References
[1]. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024 May-Jun;74(3):229-263.
[2]. Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, Falak R. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. 2022 Mar;71(3):507-526.
[3]. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022 Dec;29(1):2130-2161.
[4]. Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: A comprehensive review. Phytother Res. 2018 Nov;32(11):2109-2130.
[5]. Alizadeh SR, Ebrahimzadeh MA. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur J Med Chem. 2022 Feb 5;229:114068.
[6]. Hasan AA, Tatarskiy V, Kalinina E. Synthetic Pathways and the Therapeutic Potential of Quercetin and Curcumin. Int J Mol Sci. 2022 Nov 20;23(22):14413.
[7]. Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
[8]. Mirza MA, Mahmood S, Hilles AR, Ali A, Khan MZ, Zaidi SAA, Iqbal Z, Ge Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review. Pharmaceuticals (Basel). 2023 Nov 20;16(11):1631.
[9]. A. Massi, O. Bortolini, D. Ragno, T. Bernardi, G. Sacchetti, M. Tacchini, C. De Risi, Research progress in the modification of quercetin leading to anticancer agents, Molecules 22 (2017) 1270.
[10]. Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, Hasan M, Kumar M, Beirami SM, Tarhriz V, Soofiyani SR, Kozhamzharova L, Sharifi-Rad J, Calina D, Cho WC. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022 Aug 15;22(1):257.
[11]. Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852.
[12]. Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020 Jan;121:109604.
[13]. Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177.
[14]. Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210.
[15]. L. Cialdella-Kam, D.C. Nieman, W. Sha, M.P. Meaney, A.M. Knab, R.A. Shanely.Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults Br. J. Nutr., 109 (2013), pp. 1923-1933
[16]. Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, Hasan MM, Al Azad S, Bibi S, Hasan MN, Rahmatullah M, Chun J, Rahman MA, Kim B. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int J Mol Sci. 2022 Oct 4;23(19):11746.
[17]. Lee Y.-K., Hwang J.-T., Kwon D.Y., Surh Y.-J., Park O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010;292:228–236.
[18]. Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M, Shams MH, Baghbadorani PZ, Eskandari N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front Immunol. 2023 Feb 28;14:1077531.
[19]. Bishayee K,Ghosh S,Mukherjee A,et al. Quercetin induces cyto- chrome-C release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2 /M,in cervical carcinoma: signal cascade and drug-DNA interaction[J. Cell Proliferation,2013,46 ( 2):153 163.
[20]. He D., Guo X., Zhang E., Zi F., Chen J., Chen Q., Lin X., Yang L., Li Y., Wu W., et al. Quercetin induces cell apoptosis of myeloma and displays a synergistic effect with dexamethasone in vitro and in vivo xenograft models. Oncotarget. 2016;7:45489–45499.
[21]. Srivastava NS, Srivastava RAK. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. (2019) 52:117–28.
[22]. Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Khan AA, Rahmani AH. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules. 2021 Mar 1;26(5):1315.
[23]. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog (2013) 18(1-2):43–73.
[24]. ERDOGAN S,TURKEKUL K,DIBIRDIK I,et al. Midkine downregulation increases the efficacy of querce-tin on prostatecancer stem cell survival and mi-gration through PI3K/AKT and MAPK/ERKpathway[J].Biomedicine & Pharmacotherapy,2018,37(107):793-805.
[25]. AGHAPOUR F,MOGHADAMNIA A A,NICOLINI A,et al.Quer-cetin conjugated with silica nanoparticles inhib-its tumor growthin MCF-7 breast cancer celllines[J].Biochem Biophys Res Commun,2018,500(4):860-865.
[26]. Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Lee, W.S.; Park, O.J. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 2009, 41, 201–207.
[27]. Van der Woude, H.; Gliszczynska-Swiglo, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47.
[28]. Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193.
[29]. Rajesh R U, Sangeetha D. Therapeutic potentials and targeting strategies of quercetin on cancer cells: Challenges and future prospects. Phytomedicine. 2024 Oct;133:155902.
[30]. Lozanovski VJ, Houben P, Hinz U, Hackert T, Herr I, Schemmer P. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial) - study protocol for a randomized controlled trial. Trials. 2014 Jun 3;15:204.
[31]. Santangelo R, Silvestrini A, Mancuso C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem Toxicol. 2019 Jan;123:42-49.
[32]. Xu J, Chen HB, Li SL. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med Res Rev. 2017 Sep;37(5):1140-1185.
[33]. Patra M, Mukherjee R, Banik M, Dutta D, Begum NA, Basu T. Calcium phosphate-quercetin nanocomposite (CPQN): A multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Colloids Surf B Biointerfaces. 2017 Jun 1;154:63-73.
[34]. Karimi A, Naeini F, Asghari Azar V, Hasanzadeh M, Ostadrahimi A, Niazkar HR, Mobasseri M, Tutunchi H. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine. 2021 Jun;86:153567.
[35]. Liu ZJ, Xu W, Han J, Liu QY, Gao LF, Wang XH, Li XL. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer Drugs. 2020 Aug;31(7):684-692.
[36]. Malik A, Sultana M, Qazi A, Qazi MH, Parveen G, Waquar S, Ashraf AB, Rasool M. Role of Natural Radiosensitizers and Cancer Cell Radioresistance: An Update. Anal Cell Pathol (Amst). 2016;2016:6146595.
[37]. Li S, Zhao Q, Wang B, Yuan S, Wang X, Li K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother Res. 2018 Aug;32(8):1530-1536.
[38]. Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018 Jan;51:105-113.
Cite this article
Cai,Y. (2025). The potential anti-cancer effects of quercetin on cancer therapy. Theoretical and Natural Science,75,20-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024 May-Jun;74(3):229-263.
[2]. Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, Falak R. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. 2022 Mar;71(3):507-526.
[3]. Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022 Dec;29(1):2130-2161.
[4]. Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, Mubarak MS. Anticancer potential of quercetin: A comprehensive review. Phytother Res. 2018 Nov;32(11):2109-2130.
[5]. Alizadeh SR, Ebrahimzadeh MA. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur J Med Chem. 2022 Feb 5;229:114068.
[6]. Hasan AA, Tatarskiy V, Kalinina E. Synthetic Pathways and the Therapeutic Potential of Quercetin and Curcumin. Int J Mol Sci. 2022 Nov 20;23(22):14413.
[7]. Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
[8]. Mirza MA, Mahmood S, Hilles AR, Ali A, Khan MZ, Zaidi SAA, Iqbal Z, Ge Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review. Pharmaceuticals (Basel). 2023 Nov 20;16(11):1631.
[9]. A. Massi, O. Bortolini, D. Ragno, T. Bernardi, G. Sacchetti, M. Tacchini, C. De Risi, Research progress in the modification of quercetin leading to anticancer agents, Molecules 22 (2017) 1270.
[10]. Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, Hasan M, Kumar M, Beirami SM, Tarhriz V, Soofiyani SR, Kozhamzharova L, Sharifi-Rad J, Calina D, Cho WC. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022 Aug 15;22(1):257.
[11]. Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852.
[12]. Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020 Jan;121:109604.
[13]. Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177.
[14]. Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210.
[15]. L. Cialdella-Kam, D.C. Nieman, W. Sha, M.P. Meaney, A.M. Knab, R.A. Shanely.Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults Br. J. Nutr., 109 (2013), pp. 1923-1933
[16]. Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, Hasan MM, Al Azad S, Bibi S, Hasan MN, Rahmatullah M, Chun J, Rahman MA, Kim B. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int J Mol Sci. 2022 Oct 4;23(19):11746.
[17]. Lee Y.-K., Hwang J.-T., Kwon D.Y., Surh Y.-J., Park O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010;292:228–236.
[18]. Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M, Shams MH, Baghbadorani PZ, Eskandari N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front Immunol. 2023 Feb 28;14:1077531.
[19]. Bishayee K,Ghosh S,Mukherjee A,et al. Quercetin induces cyto- chrome-C release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2 /M,in cervical carcinoma: signal cascade and drug-DNA interaction[J. Cell Proliferation,2013,46 ( 2):153 163.
[20]. He D., Guo X., Zhang E., Zi F., Chen J., Chen Q., Lin X., Yang L., Li Y., Wu W., et al. Quercetin induces cell apoptosis of myeloma and displays a synergistic effect with dexamethasone in vitro and in vivo xenograft models. Oncotarget. 2016;7:45489–45499.
[21]. Srivastava NS, Srivastava RAK. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. (2019) 52:117–28.
[22]. Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Khan AA, Rahmani AH. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules. 2021 Mar 1;26(5):1315.
[23]. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog (2013) 18(1-2):43–73.
[24]. ERDOGAN S,TURKEKUL K,DIBIRDIK I,et al. Midkine downregulation increases the efficacy of querce-tin on prostatecancer stem cell survival and mi-gration through PI3K/AKT and MAPK/ERKpathway[J].Biomedicine & Pharmacotherapy,2018,37(107):793-805.
[25]. AGHAPOUR F,MOGHADAMNIA A A,NICOLINI A,et al.Quer-cetin conjugated with silica nanoparticles inhib-its tumor growthin MCF-7 breast cancer celllines[J].Biochem Biophys Res Commun,2018,500(4):860-865.
[26]. Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Lee, W.S.; Park, O.J. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 2009, 41, 201–207.
[27]. Van der Woude, H.; Gliszczynska-Swiglo, A.; Struijs, K.; Smeets, A.; Alink, G.M.; Rietjens, I.M. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003, 200, 41–47.
[28]. Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193.
[29]. Rajesh R U, Sangeetha D. Therapeutic potentials and targeting strategies of quercetin on cancer cells: Challenges and future prospects. Phytomedicine. 2024 Oct;133:155902.
[30]. Lozanovski VJ, Houben P, Hinz U, Hackert T, Herr I, Schemmer P. Pilot study evaluating broccoli sprouts in advanced pancreatic cancer (POUDER trial) - study protocol for a randomized controlled trial. Trials. 2014 Jun 3;15:204.
[31]. Santangelo R, Silvestrini A, Mancuso C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem Toxicol. 2019 Jan;123:42-49.
[32]. Xu J, Chen HB, Li SL. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med Res Rev. 2017 Sep;37(5):1140-1185.
[33]. Patra M, Mukherjee R, Banik M, Dutta D, Begum NA, Basu T. Calcium phosphate-quercetin nanocomposite (CPQN): A multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Colloids Surf B Biointerfaces. 2017 Jun 1;154:63-73.
[34]. Karimi A, Naeini F, Asghari Azar V, Hasanzadeh M, Ostadrahimi A, Niazkar HR, Mobasseri M, Tutunchi H. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine. 2021 Jun;86:153567.
[35]. Liu ZJ, Xu W, Han J, Liu QY, Gao LF, Wang XH, Li XL. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer Drugs. 2020 Aug;31(7):684-692.
[36]. Malik A, Sultana M, Qazi A, Qazi MH, Parveen G, Waquar S, Ashraf AB, Rasool M. Role of Natural Radiosensitizers and Cancer Cell Radioresistance: An Update. Anal Cell Pathol (Amst). 2016;2016:6146595.
[37]. Li S, Zhao Q, Wang B, Yuan S, Wang X, Li K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother Res. 2018 Aug;32(8):1530-1536.
[38]. Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018 Jan;51:105-113.









