1. Introduction
Alzheimer’s disease: Alzheimer’s disease (AD), a kind of widespread and severe neurodegenerative disease, is marked by a steady decrease in memory and cognitive capacities with featuring pathological characteristics and two main features that were discussed most often are the external accumulation of β-amyloid (Aβ) and Phosphorylated-Tau protein (p-Tau) on the cortex of mammals, also they are the reasons of AD that have been accepted widely. Factors of AD contributing to risk encompass infections, ensuing cellular stress, or neuroinflammation, all of which act as triggers for AD. As an illustration, it has been established that the Zika virus can invade neurons, potentially harming the central nervous system and increase the culminating rate in AD [1-4].
ER stress: The endoplasmic reticulum (ER) is an organelle which in most of the eukaryotes that serves as a hub for creation, folding, and post-translational alteration of proteins which are secretory and attached to membranes. Disruptions in protein folding within the ER will lead to ER stress and trigger a series of signaling routes which are known as the unfolded protein response (UPR). ER stress is characterized by the accumulation of non-folded or improperly folded proteins in the ER, disrupting both the ER and cellular equilibrium. Stress in the ER triggers the unfolded protein response (UPR), a sequence of signaling routes originating from three ER transmembrane proteins—IRE1α, PERK, and ATF6. Under ER stress, activation of all three receptors will take place through cleavage (like ATF6) or phosphorylation (like PERK and IRE1) to preserve the ER in balance state. Alterations in protein structure and misfolding act as pivotal occurrences of the pathophysiology of AD. Consequently, stress in ER seems to be a clear origin of AD.
BiP Inducer X: BiP Inducer X (BIX) is proved to be a useful ER stress inhibitor [5], it selectively stimulated BiP, along with minor activations of calreticulin, C/EBP homologous protein and 94 kDa glucose-regulated protein (GRP94). The stimulation of BIX to BiP mRNA occurred via the ER stress response activation components preceding the BiP gene, utilizing the ATF6 (activating transcription factor 6) pathway. In this experiment, the chemical compound named 1-(3,4-dihydroxy-phenyl)-2-thiocyanate-ethanone is used as BiX.
Activating transcription factor 6: ATF6 is a kind of UPR sensor and a basic leucine zipper transcription factor activated under ER stress. It serves as one of primary quality control mechanism in cells to enhance protein processing and reestablish ER homeostasis.
ZIKV: Zika virus (ZIKV) is an infectious virus that belongs to the Flavivirus genus. It is an arbovirus that causes congenital malformations. ZIKV proteins is mainly distributed near and replicates within the receptor and induces a massive vacuolization and interference with apoptosis-related receptor pathways. In this experiment, we use ZIKV to induce ER stress to increase AD features.
Brain organoid: Brain organoids resemble the characteristics of different regions of the brain, also provide opportunities to study the development of AD and investigate the details about mechanisms of AD and also other neurodegenerative diseases. In order to investigate the causes of AD phenotypes in molecular aspect after ZIKV infection, 3D structure in brain organoid systems will be used to study the AD development mechanism after ZIKV through observing mechanisms in the brain organoid. In this experiment, the human-induced pluripotent stem cells (iPSCs) will be used to gain brain organoid models.
2. Method
Generate brain organoids: Use human-induced pluripotent stem cells (iPSCs) to generation brain organoid models. Two types of iPSCs: the wild-type (WT) and AD patient-derived will be used to generate two group which WT is control group and AD patient-derived is experiment group. Both of the two kinds of brain organoid are produced with similar process which is described by the previous brain organoid developing method that was developed and mentioned by an article written by Lancaster et al [6].
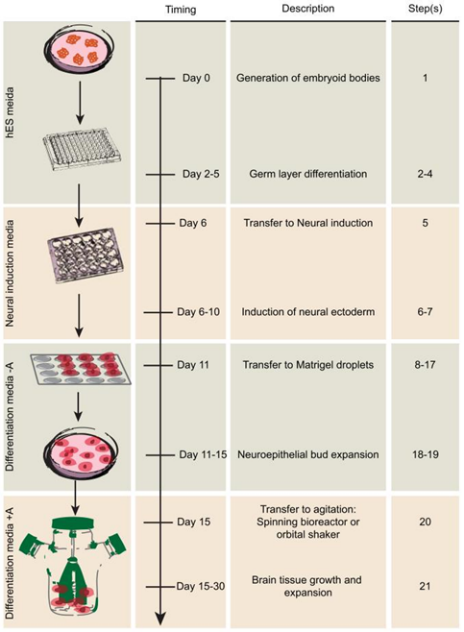
Figure 1. the process of brain organoid production
Simply put, induced pluripotent stem cells are broken down into individual cells on day 0 of cell culture. The iPSC colonies will be placed in one of six wells that will be used to make embryoid bodies (EBs). After 5-7 days, Small EBs with clear boundaries can be seen under a microscope. EB continued to be cultured in incubator under 37 °C and 5% concentration of CO2. After EB is larger than 500 μm , EB will be moved to a 24-well plate that is low adhesion and seeded them into the DMEM-F12 medium, with the addition of 1X N2 supplement (Gibco), heparin solution of 1 μg/ml concentration, 1X Gluta-MAX (Gibco), and 1X MEM-NEAA (Gibco). Then after 6 days culturing inside the induction media, the neurons that are being transferred will be transferred to Matrigel (Corning) droplets. The droplets are incubated at 37 °C for 20 mins, then isolate them from the Parafilm and culture inside cerebral organoid differentiation media consisting of a 1: 1 mixture of DMEM/F12: neuro-based medium supplemented with 1X N2 supplement (Gibco), 1X B27 Vitamin A Free Supplement (Gibco), 2-mercaptoethanol,1X insulin (Sigma), 1X GlutaMAX (Gibco) and 1X MEM-NEAA. After four days of steady culturing, the medium should be changed by new ones and the organic matter in CODM are transferred to the orbital oscillator via 2-mercaptoethanol, 1X N2 supplement (Gibco), 1X B27 supplementation Vitamin A (Gibco), 1X glutathione (Gibco), 1X insulin (Sigma) and 1X MEM-NEAA (Gibco). Then brain organoid will form clear structure after 40th day. The final production of brain organoid will be divided into two groups and every group involve a large amount of brain organoid individual to avoid experimental errors.
Infected with ZIKV: At the 60th day of brain organoid development, the brain organoid is treated with ZIKV for 24 hours then the medium will be replaced by CODM which is virus-free to avoid further infection and errors. The brain organoids will be kept inside the CODM with the medium is changed by new ones for 1 day interval during the whole experiment. In previous articles, ZIKV have been proved to be able to accelerate the development of the levels of Aβ and p-Tau which are in AD organoids as well as WT organoids [3].
Use BiP Inducer X as ER stress inhibitor: Use pre-experiment to identity the suitable concentration of BiP Inducer X. According to the article [7], we can assume that the treatment of cells is with 50 μM BIX since under this concentration will cause the largest induction of BiP and smallest toxicity to brain cells. The ZIKV-infected brain organoid is exposed in BIX for 1h 2h 4h 8h 12h and 24h. Then the immunostaining is used to get the image at every time periods. The aβ accumulation and p-tau level will be measured as the characteristic of AD to see whether the AD have been relieved.
Immunostaining and imaging: In this experiment use immunostaining to get image of the distribution of aβ and p-tau in brain organoid. The Immunostaining will be used at the time of before infection by ZIKV, after 24h treat with ZIKV and after every time point after exposing in BIX. The details of immunostaining will be listed below. For each time point and each group of the brain organoids, they will be fixed for one night and use 4% paraformaldehyde polyformaldehyde through immunofluorescence staining under temperature of 4 °C. After greater than three washes of Phosphate Buffered Saline (PBS), the organoids will be mixed into 15-ml tubes with 30% concentrated sucrose solution, then incubate overnight, then add 10% concentrated gelatin and 30% concentrated sucrose solution and send for deep-frozen. brain organoids will be dried after cleaning for a few times with PBS. Organics should be blocked for 1 hour under PBS and room temperature with 5% concentration of goat serum and TritonX-100 with 0.5% concentration. After blockade, slides were treated at 4 ° C at night with a rabbit anti-cleaved caspase-3 antibody (1:8,000). After being washed with PBS, slides will be cultured with biotin-bound goat secondary antibodies against rabbits (1:1,000) for about one hour under room temperature, then wash it using PBS again. Avidin-biotin-peroxidase complex will be well-prepared. The slides will be incubated inside the complex for another one hour under room temperature. Slides will be made using 3.3% diaminobenzidine (DAB) (Sigma-Aldrich, Inc.) and 0.05% H2O2 on PBS after being rinsed.
3. Discussion
In this experiment the ZIKV-infected brain organoid is treated by ER stress inhibitor BIX, the result could be the aβ accumulation and p-tau level have been reduced since the previous article about BIX have proved to be reduce ER stress in chemical induced brain [7], so we can assume the BIX also works in AD brain organoid induced by ZIKV. If the inhibition of ATF6 pathway is proved by the experiment that is able to reduce ER stress then make aβ and p-tau less. Then BIX maybe able to be considered to use as a treatment of AD in the future.
There are still some disadvantages about this experiment design. The experiment only used brain organoid to find the impact of BIX which have many limitations. The further experiment could be raised to check the reliability of the result of the experiment mentioned in this paper.
For example, the experiment about exploration of the treatment of BIX on other virus induced AD such as herpes simplex virus (HSV),Japanese encephalitis virus (JEV), and cytomegalovirus (CMV) which can also infect neurons just like ZIKV.
Other pathways are also able to be explored to find the treatment of AD in different aspect. The further experiment could focus on IRE1α pathway to investigate whether the ER stress inhibitor through IRE1α pathway would reduce AD.
The brain organoid also has some limitation, so animal system like mice or more complicated brain organoid model which is described in article [8] should be used to explore the effect of BIX in a more complex system and identify whether there is side effect on treatment of BIX to access the possibility of act as a treatment.
4. Conclusion
The article have mainly discussed a possibility about the treatment of AD through ATF6 pathway by using specific inhibitor(BIX),the brain organoid is used in experiment design to provide the model of ZIKV infection and AD . The immunostaining and image is used to get distribution of the AD feature Aβ and p-tau and how they accumulate. The experiment could be improved in few steps if the better and easier operation could be achieved in actual experiment.
References
[1]. Trejo-Lopez JA, Yachnis AT, Prokop S. Neuropathology of Alzheimer's Disease. Neurotherapeutics. 2022 Jan;19(1):173-185. doi: 10.1007/s13311-021-01146-y. Epub 2021 Nov 2. PMID: 34729690; PMCID: PMC9130398.
[2]. joolabady A, Lindholm D, Ren J, Pratico D. ER stress and UPR in Alzheimer's disease: mechanisms, pathogenesis, treatments. Cell Death Dis. 2022 Aug 15;13(8):706. doi: 10.1038/s41419-022-05153-5. PMID: 35970828; PMCID: PMC9378716.
[3]. Lee SE, Choi H, Shin N, Kong D, Kim NG, Kim HY, Kim MJ, Choi SW, Kim YB, Kang KS. Zika virus infection accelerates Alzheimer's disease phenotypes in brain organoids. Cell Death Discov. 2022 Apr 2;8(1):153. doi: 10.1038/s41420-022-00958-x. PMID: 35368019; PMCID: PMC8976422.
[4]. Bruno F, Abondio P, Bruno R, Ceraudo L, Paparazzo E, Citrigno L, Luiselli D, Bruni AC, Passarino G, Colao R, Maletta R, Montesanto A. Alzheimer's disease as a viral disease: Revisiting the infectious hypothesis. Ageing Res Rev. 2023 Nov; 91: 102068. doi: 10.1016/j.arr.2023.102068. Epub 2023 Sep 12. PMID: 37704050.
[5]. Ha TK, Hansen AH, Kildegaard HF, Lee GM. BiP Inducer X: An ER Stress Inhibitor for Enhancing Recombinant Antibody Production in CHO Cell Culture. Biotechnol J. 2019 Oct;14(10): e1900130. doi: 10.1002/biot.201900130. Epub 2019 Jul 5. PMID: 31161665.
[6]. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014 Oct;9(10):2329-40. doi: 10.1038/nprot.2014.158. Epub 2014 Sep 4. PMID: 25188634; PMCID: PMC4160653.
[7]. Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008 Feb;15(2):364-75. doi: 10.1038/sj.cdd.4402276. Epub 2007 Nov 30. PMID: 18049481.
[8]. Wörsdörfer P, Dalda N, Kern A, Krüger S, Wagner N, Kwok CK, Henke E, Ergün S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019 Oct 30;9(1):15663. doi: 10.1038/s41598-019-52204-7. PMID: 31666641; PMCID: PMC6821804.
Cite this article
Zhang,Y. (2025). Use ER stress inhibitor BiP Inducer X in ZIKV-infected brain organoid to see if reducing ER stress through ATF6 pathway can alleviate pathological feature of Alzheimer’s disease. Theoretical and Natural Science,76,85-89.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Trejo-Lopez JA, Yachnis AT, Prokop S. Neuropathology of Alzheimer's Disease. Neurotherapeutics. 2022 Jan;19(1):173-185. doi: 10.1007/s13311-021-01146-y. Epub 2021 Nov 2. PMID: 34729690; PMCID: PMC9130398.
[2]. joolabady A, Lindholm D, Ren J, Pratico D. ER stress and UPR in Alzheimer's disease: mechanisms, pathogenesis, treatments. Cell Death Dis. 2022 Aug 15;13(8):706. doi: 10.1038/s41419-022-05153-5. PMID: 35970828; PMCID: PMC9378716.
[3]. Lee SE, Choi H, Shin N, Kong D, Kim NG, Kim HY, Kim MJ, Choi SW, Kim YB, Kang KS. Zika virus infection accelerates Alzheimer's disease phenotypes in brain organoids. Cell Death Discov. 2022 Apr 2;8(1):153. doi: 10.1038/s41420-022-00958-x. PMID: 35368019; PMCID: PMC8976422.
[4]. Bruno F, Abondio P, Bruno R, Ceraudo L, Paparazzo E, Citrigno L, Luiselli D, Bruni AC, Passarino G, Colao R, Maletta R, Montesanto A. Alzheimer's disease as a viral disease: Revisiting the infectious hypothesis. Ageing Res Rev. 2023 Nov; 91: 102068. doi: 10.1016/j.arr.2023.102068. Epub 2023 Sep 12. PMID: 37704050.
[5]. Ha TK, Hansen AH, Kildegaard HF, Lee GM. BiP Inducer X: An ER Stress Inhibitor for Enhancing Recombinant Antibody Production in CHO Cell Culture. Biotechnol J. 2019 Oct;14(10): e1900130. doi: 10.1002/biot.201900130. Epub 2019 Jul 5. PMID: 31161665.
[6]. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014 Oct;9(10):2329-40. doi: 10.1038/nprot.2014.158. Epub 2014 Sep 4. PMID: 25188634; PMCID: PMC4160653.
[7]. Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008 Feb;15(2):364-75. doi: 10.1038/sj.cdd.4402276. Epub 2007 Nov 30. PMID: 18049481.
[8]. Wörsdörfer P, Dalda N, Kern A, Krüger S, Wagner N, Kwok CK, Henke E, Ergün S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019 Oct 30;9(1):15663. doi: 10.1038/s41598-019-52204-7. PMID: 31666641; PMCID: PMC6821804.









