1. Introduction
Postpartum depression (PPD) is a common mental health issue worldwide, affecting approximately 10% to 15% of postpartum women, with significant and lasting impacts on both maternal and infant health [1]. Despite the importance of antidepressant medications in alleviating PPD symptoms, their use in breastfeeding women has raised widespread concerns about the potential impact of drug transfer through breast milk on infant health [2, 3]. Existing research has primarily focused on the mechanisms of drug transfer and permeability, yet there remain notable gaps, particularly regarding the differences in permeability among different antidepressants, the long-term health effects of drug combinations on infants, and the role of individual differences in drug transfer [4]. As a result, this paper provides a comprehensive review of the existing literature to evaluate the safety of various antidepressants during breastfeeding, with a particular focus on their transfer into breast milk and the potential risks to infant health. Through a comparison of studies on selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), it offers personalized and safe medication recommendations for clinical practice. It can be argued that the paper helps to provide clinicians with safer medication guidance and develop more appropriate treatment plans for breastfeeding women.
2. Overview of Postpartum Depression
2.1. Definition and Characteristics of Postpartum Depression
PPD refers to the onset of severe depressive symptoms in women following childbirth, typically occurring within 4 to 6 weeks postpartum. In contrast to temporary mood swings or the baby blues, PPD is a persistent and severe mental health condition that can significantly impact both maternal and infant well-being. The clinical manifestations of PPD include persistent low mood, disinterest in the baby or feelings of inadequacy in the maternal role, anxiety, insomnia, appetite changes, low energy, and diminished self-esteem. In severe cases, PPD may be accompanied by suicidal thoughts or behaviors, posing a great risk to both maternal and infant health [5]. PPD symptoms usually appear within the first six months postpartum and meet the diagnostic criteria for major depressive disorder, impairing the mother’s daily functioning and influencing the infant’s health. Unlike the typical short-term emotional changes after childbirth, PPD is often persistent, requiring professional intervention. If untreated, it can lead to long-term psychological issues and heightened risks to both maternal and infant health [6].
2.2. Factors Behind Postpartum Depression
The risk factors for PPD are multifaceted, involving a complex interplay of psychological, social, neuroendocrine, epigenetic, and genetic factors, with stress and adverse life events playing key roles. Women who experience significant stress or adverse life events, such as sexual abuse, major loss, or chronic negative experiences, are at a heightened risk of developing postpartum depression [7]. In addition, environmental factors such as prenatal depression or anxiety, disruptions in mother-infant interactions, lack of social support, economic hardship, and marital stress can elevate the risk of PPD [8]. From a neuroendocrine perspective, dysregulation of the HPA axis and fluctuations in reproductive hormones, such as estradiol and progesterone, may affect postpartum mood states by influencing neuroinflammation and neurotransmitter signaling pathways[9]. Moreover, epigenetic changes, such as DNA methylation of the OXTR gene, along with alterations in neuroinflammation and neurotransmitter levels, have been strongly linked to postpartum depression [10]. Genetically, polymorphisms in specific genes, such as the ESR1 gene and serotonin transporter gene, are linked to an increased risk of postpartum depression [11]. These interrelated factors collectively contribute to the heightened risk of postpartum depression, suggesting that its onset results from the interplay of biological, psychological, and social influences [1].
3. Mechanism of Antidepressants in the Treatment of Postpartum Depression
3.1. Major Antidepressants Used for Postpartum Depression
Antidepressant medications are typically classified into five categories based on on how they work: SSRIs, SNRIs, tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), as well as atypical antidepressants [12]. In particular, SSRIs and SNRIs are the first-line treatments for PPD, while MAOIs and TCAs are less frequently prescribed due to their notable side effects [6]. Drugs transfer into breast milk primarily via passive diffusion of unbound, non-ionized drug molecules. This process can be affected by factors such as the drug’s physicochemical properties, the mother’s physiological condition, and the composition of breast milk, such as protein and lipid content) [2]. Thus, breast milk is not a simple reservoir for drugs but a site of bidirectional substance transfer. Among SSRIs, common drugs like paroxetine and sertraline have relatively low concentrations in breast milk, which are generally considered to have minimal impact on infants, making them widely used for treating postpartum depression during breastfeeding [13]. However, despite their relative safety, close monitoring of the infant is recommended to ensure the absence of adverse reactions. In contrast, SSRIs like fluoxetine have higher concentrations in breast milk, requiring special caution when used [2]. And SNRIs, such as venlafaxine and duloxetine, work by inhibiting the reuptake of serotonin and norepinephrine, enhancing neurotransmitter activity. These medications also pass into breast milk. Venlafaxine is generally considered safe due to its low concentration in breast milk, although it may occasionally cause mild sleep disturbances or gastrointestinal discomfort in infants [8]. Duloxetine has a low concentration in breast milk and is generally regarded as safe for both mother and infant [14]. Although SSRIs and SNRIs are commonly used in the treatment of PPD, the choice of medication should still be individualized based on specific circumstances. In clinical practice, healthcare providers should balance the therapeutic benefits of the medication with its potential impact on breastfeeding, to ensure the safety of both mother and infant [6]. Furthermore, treatment plans should be adjusted based on the severity of postpartum depression and the mother’s health status to maximize both efficacy and safety [4].
3.2. Antidepressant Transfer to Breast Milk in Postpartum Depression Treatment
The transfer of antidepressants into breast milk during the treatment of PPD is affected by multiple factors. In particular, the physicochemical properties of the drug, like molecular size, lipophilicity, and hydrophilicity, determine whether the drug can pass through the mammary ducts into the milk. Lipophilic drugs tend to accumulate more readily in breast milk, while hydrophilic drugs are less able to traverse the lipid-rich layers of the mammary gland [1]. Besides, the drug’s binding affinity to plasma proteins plays a key role in its concentration in breast milk, as unbound or free drug molecules are more likely to transfer into the milk [14]. And the metabolism of the drug also plays a critical role in its transfer into breast milk. Metabolic products crated during maternal metabolism may have various pharmacological effects, and in some cases, these metabolites may be transferred to the infant through breast milk [1]. Moreover, an increase in mammary blood flow may accelerate drug transfer, particularly for acidic drugs, which can result in higher concentrations in breast milk [2]. The transfer of antidepressants into breast milk mainly depends on the drug’s physicochemical properties, plasma protein binding rate, and metabolic characteristics. The drug’s biological half-life also influences its accumulation in breast milk, with drugs that have a longer half-life potentially accumulating in higher concentrations, thereby increasing the risk of infant exposure [2]. Therefore, when selecting antidepressants, it is crucial to consider the drug's milk transfer properties to ensure the safety of both mother and infant [4].
3.3. Measurement of Antidepressant Concentrations in Breast Milk
The determination of antidepressant concentrations in breast milk is key to assess the potential impact of maternal medication on infant health. There are significant differences in the permeability of various antidepressants into breast milk, which are influenced not only by the molecular structure and lipophilicity of the drug but also by the accumulation of drug metabolites in milk. For instance, venlafaxine and citalopram exhibit relatively high permeability, with milk-to-plasma ratios of 2.59 and 2.22, respectively [1], indicating that they may have a greater impact on the infant. In contrast, fluvoxamine and fluoxetine show lower permeability, suggesting that their use during breastfeeding may be relatively safer [14]. In umbilical cord plasma, nortriptyline presents a higher permeability (2.97), whereas bupropion has a permeability ratio of 1.14 [2], reflecting significant differences in drug transfer characteristics across various body fluids. For a more comprehensive understanding of antidepressant transfer into breast milk, in vitro models and animal studies provide valuable insights. For example, by using the Henderson-Hasselbalch equation and pH distribution models, the drug transfer process can be modeled more accurately [15]. Table 1 summarizes data from animal studies on antidepressant secretion into milk, thus providing a basis for further extrapolation to human drug transfer. Notably, pig models are often used due to the similarity in milk composition between pigs and humans, making them highly valuable for studying milk drug transfer [16].
Table 1: Animal Studies on Antidepressant Drug Secretion into Breast Milk
Drug | Drug Class | Animal Species | M/P Ratio |
Agomelatine | Melatonergic and Serotonergic Agonist# | Rat | 0.348-1.128 |
Moclobemide | MAOI | Mouse | 1.41 |
Trazadone | SARI | Mouse | 0.20 |
Escitalopram | SSRI | Rat | \( \lt \) 0.15* |
Paroxetine | SSRI | Rat | \( \lt \) 0.028* |
Fluoxetine | SSRI | Rat | \( \lt \) 0.028* |
Venlafaxine | SNRI | Rat | \( \lt \) 0.0578* |
*M /P ratio was not available. The listed value is $erum Cone Ratio (Pup/Dam), which was calculated by using the drug concentration in the pup's serum divided by that in the dam's serum on day 7 of drug exposure. Medianvalues for pup and dam serum on day 7 were averaged to obtain this value, and only data from litters l and2 were utilized. | |||
In addition, Table 2 consolidates clinical research data on the concentrations of antidepressants in breast milk during lactation, offering essential guidance for clinical decision-making. Specifically, SSRIs like citalopram and sertraline have lower milk-to-plasma ratios and are typically considered the first-line treatment for PPD [6]. However, SNRIs like venlafaxine show lower concentrations in breast milk, yet their metabolite, desvenlafaxine, exhibits higher levels, indicating that even if the maternal drug concentration is low, its metabolites may still affect the infant [8].
Table 2: Observed Adverse Effects of Antidepressants in Infants During Lactation
Drug | First-Line/Second-Line Treatment | Study Type | Obs. M/P Ratio | Observed Adverse Effects in Infants |
Amitriptyline | Second Line | Cohort Study | Mean: 0.9 | One infant tested in the low normal range for development and was slightly hypotonic |
Bupropion | First Line | Cohort Study | Mean: 2.8 | No adverse effects reported |
Citalopram | First Line | Cohort Study | Mean: 1.8 | No adverse effects reported |
Clomipramine | Second Line | Cohort Study | 0.4-3.0 | No adverse effects reported |
Desipramine | Second Line | Cohort Study | Mean: 1.45 | No adverse effects reported |
Desvenlafaxine | First Line | Cohort Study | Mean: 2.24 | 7 infants were at a lower growth percentile but all 10 infants had normal development |
Despite the relatively low milk transfer for most SSRIs and SNRIs, caution is still advised when prescribing these medications during breastfeeding, and regular monitoring of infant drug levels is essential. The permeability ratios of antidepressants in umbilical cord plasma, as shown in Figure 1, reflect the transport characteristics of different drugs, which inform the risk assessment of infant drug exposure. With these findings, clinicians will be able to make informed decisions based on the concentrations of drugs in breast milk and the potential infant exposure, minimizing potential risks to the infant [4].
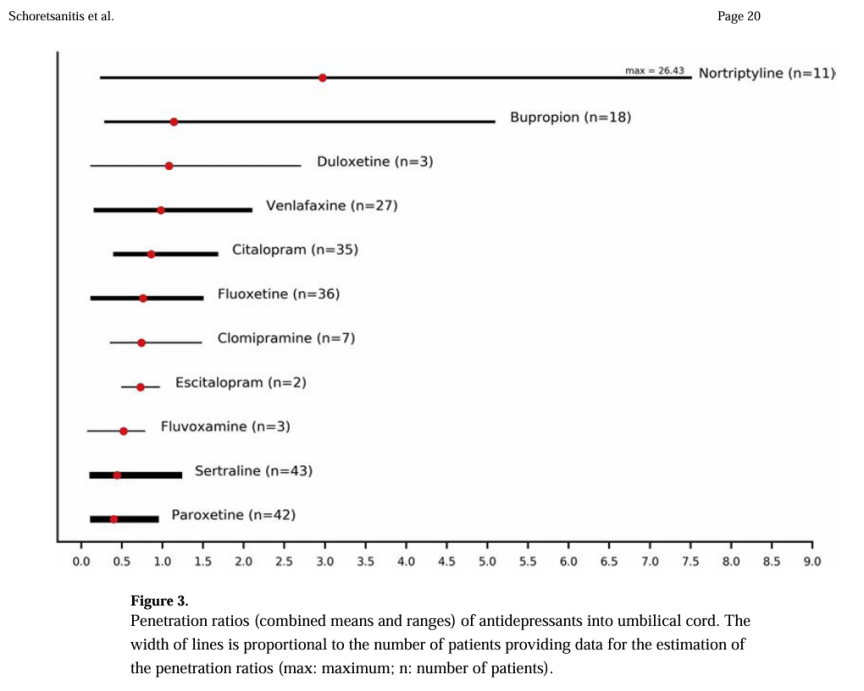
Figure 1: Umbilical Cord Antidepressant Penetration Ratios (Means and Ranges), Line Width Proportional to Sample Size (Max: Maximum; n: Patients)
4. The Impact on Maternal and Infant Health
4.1. The Impact of Antidepressants on Breast Milk Production
Antidepressants, particularly SSRIs, may delay lactation onset. Studies indicate that mothers on SSRIs had a delayed onset of milk secretion by about 16.7 hours, thus increasing the risk of delayed breastfeeding initiation[4]. This delay may be related to the impact of SSRIs on prolactin levels, a hormone critical for milk production. However, the delay is often temporary and does not greatly affect breastfeeding in the early postpartum period [8]. Still, mothers using SSRIs during the third trimester were less likely to initiate breastfeeding. A retrospective study shows that mothers who took antidepressants are 37% less likely to initiate breastfeeding at the time of discharge compared to those who did not use medications [8]. Other studies have also suggested that SSRIs may lead to earlier cessation or delayed initiation of breastfeeding, particularly during the third trimester of pregnancy [6]. A study involving 2,859 women indicated that mothers who used SSRIs throughout pregnancy were 37% less likely to breastfeed compared to non-users, while those who used SSRIs during the third trimester had a 75% lower chance of breastfeeding [8]. Beyond medication, factors such as maternal health, support systems, and socioeconomic conditions also play a crucial role in breastfeeding success. For example, effective management of maternal depression may improve the likelihood of successful breastfeeding [4]. The impact of venlafaxine on breast milk production shows considerable variability. Some studies have suggested that venlafaxine may lead to increased prolactin levels, potentially causing symptoms like galactorrhea [8]. Case reports have indicated that mothers taking venlafaxine experienced prolonged milk ejection reflex, suggesting that the medication may initially affect lactation [9]. Venlafaxine is also considered a potential contributor to elevated prolactin levels, which can lead to lactation-related symptoms like galactorrhea [2]. In some cases, venlafaxine combined with other medications has resulted in a significant reduction in milk production. For example, a woman with long-term depression who took venlafaxine during pregnancy and started breastfeeding found that adding aripiprazole to her treatment regimen led to a substantial decrease in milk supply, which ultimately ceased within 21 days [6].
4.2. The Impact of Antidepressants on Infant Neurodevelopment and Growth
Escitalopram has minimal impact on the neuropsychological development of infants. For example, one study found that infants whose mothers took an average dose of 199 μg/kg/day of escitalopram scored 110% on the Denver Developmental Scale, with no significant developmental abnormalities [2]. During maternal escitalopram use, infants show normal weight gain and no significant effects on neuropsychological development [13]. However, at higher doses or when combined with other medications, escitalopram may have negative effects on infants. For instance, one infant developed irritability, vomiting, fever, and elevated liver enzymes during maternal escitalopram use, which resolved after discontinuation [8]. Moreover, some infants may exhibit feeding behavior changes, such as persistent high-pitched crying, especially within two hours of breastfeeding, with symptoms improving as the mother adjusted her medication dose [1]. TThese instances suggest that infants may experience temporary feeding or behavioral issues, particularly at higher escitalopram doses. Escitalopram use may increase the risk of neonatal adaptation syndrome, particularly in breastfed infants, who are three times more likely to develop PNA than formula-fed infants [8]. Most infants show normal neurodevelopment and weight gain during maternal escitalopram use, but higher doses or long-term use may lead to serious side effects, including seizures, elevated liver enzymes, and bruxism [1].
Most studies on venlafaxine show no significant neurodevelopmental abnormalities or weight gain issues in infants. Infants of mothers on venlafaxine typically exhibit normal weight gain and pass Denver developmental screenings [2]. However, at high doses, venlafaxine may negatively affect infants. For instance, an infant whose mother took 375 mg/day developed symptoms like drowsiness, feeding difficulties, and dehydration, possibly due to withdrawal effects [8]. Besides, some studies reported that higher doses of venlafaxine may lead to increased drowsiness and poor alertness in infants [9]. It may also induce withdrawal symptoms in infants, including hypoglycemia, bradycardia, irritability, and hypothermia [8]. And these symptoms usually improve after stopping breastfeeding, suggesting that the drug in breast milk may may affect their severity [9]. However, most studies suggest that venlafaxine use during pregnancy and breastfeeding does not result in long-term developmental issues or growth abnormalities in infants [8]. Venlafaxine has a relatively small impact on infants, with most showing normal growth and neurodevelopment during maternal use, though high doses of venlafaxine may lead to drowsiness, feeding difficulties, and withdrawal symptoms, especially when mothers use higher doses [1]. Thus, close monitoring of the infant’s health is necessary when using venlafaxine, particularly at high doses [4].
5. Conclusion
Breastfeeding women should exercise caution when using antidepressants, as different medications vary significantly in how they permeate into breast milk. The result demonstrate that venlafaxine, mirtazapine, and escitalopram have higher concentrations in breast milk, which may pose potential risks to infants, while reboxetine, trazodone, and amoxapine have lower excretion levels, making them relatively safer for use. Despite some medications having low concentrations in breast milk, there are reports indicating that they may still have adverse effects on infant health. Thus, clinicians prescribing antidepressants to breastfeeding mothers should carefully consider the M/P ratio, so as to minimize risks to the infant. Future research should focus on investigating the long-term health effects of these medications on infants, ensuring the protection of both maternal treatment and infant safety and health.
References
[1]. Schoretsanitis, G., et al. (2021) Antidepressant transfer to amniotic fluid, cord blood, and breast milk: a systematic review and comprehensive analysis. Prog neuropsychopharmacology Biological psychiatry.
[2]. Weissman, A.M., et al. (2004) Pooled analysis of antidepressant levels in nursing mothers, breast milk and nursing infants. American Journal of Psychiatry, 161(6): 1066-1078.
[3]. Schoretsanitis, G., et al. (2020). Effects of pregnancy on antidepressant pharmacokinetics: a systematic critical review and meta-analysis. Expert Opin medication Metab Toxicol, 16(5): 431-440.
[4]. Deligiannidis, K.M., et al. (2023) Zuranolone for postpartum depression. American Journal of Psychiatry, 180(9) : 668-675.
[5]. WHO. (20013) WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva: World Health Organization.
[6]. Hale, T.W., et al. (2010) Discontinuation syndrome in newborns whose mothers took antidepressants while pregnant or breastfeeding. Breastfeed Med,5:283-288.
[7]. Payne, J.L. and Maguire, J. (2018) Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol, 52: 165-180.
[8]. Grzeskowiak, L., et al. (2022) Perinatal antidepressant use and breastfeeding outcomes: Findings from the Norwegian Mother, Father and Child Cohort Study. Acta Obstet Gynecol Scand, 101: 344-354.
[9]. Venkatesh, K., et al. (2017) Impact of antidepressant treatment during pregnancy on obstetric outcomes among women previously treated for depression: An observational cohort study. J Perinatol, 37: 1003-1009.
[10]. Hendrick, V., et al. (2001) Venlafaxine and breast-feeding. Am J Psychiatry.
[11]. Uguz, F. (2021) A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther, 28: e118-e126.
[12]. Desaunay, P., et al. (2023) Benefits and risks of antidepressants during pregnancy: a systematic review of a meta-analysis. Pediatric drugs, 25(3): 247-265.
[13]. Berle, J.Ø., et al. (2004) Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: infant exposure, clinical symptoms, and cytochrome p450 genotypes. J Clin Psychiatry. 65(9): 1228-1234.
[14]. Pons, G., Rey, E. and Matheson, I. (1994) Excretion of psychoactive drugs into breast milk. Pharmacokinetic principles and recommendations. Clin Pharmacokinet. 27(4): 270-289.
[15]. Kieviet, N., et al. (2015) Risk factors for poor neonatal adaptation after exposure to antidepressants in utero. Acta Paediatr, 104: 384-391.
[16]. Kronenfeld, N., et al. (2018) Chronic use of psychotropic medications in breastfeeding women: Is it safe? PLoS One, 13: e0197196.
Cite this article
Cao,J. (2025). Impact of Antidepressant Medication on Lactating Women and Infant Health. Theoretical and Natural Science,96,40-46.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Schoretsanitis, G., et al. (2021) Antidepressant transfer to amniotic fluid, cord blood, and breast milk: a systematic review and comprehensive analysis. Prog neuropsychopharmacology Biological psychiatry.
[2]. Weissman, A.M., et al. (2004) Pooled analysis of antidepressant levels in nursing mothers, breast milk and nursing infants. American Journal of Psychiatry, 161(6): 1066-1078.
[3]. Schoretsanitis, G., et al. (2020). Effects of pregnancy on antidepressant pharmacokinetics: a systematic critical review and meta-analysis. Expert Opin medication Metab Toxicol, 16(5): 431-440.
[4]. Deligiannidis, K.M., et al. (2023) Zuranolone for postpartum depression. American Journal of Psychiatry, 180(9) : 668-675.
[5]. WHO. (20013) WHO Recommendations on Postnatal Care of the Mother and Newborn. Geneva: World Health Organization.
[6]. Hale, T.W., et al. (2010) Discontinuation syndrome in newborns whose mothers took antidepressants while pregnant or breastfeeding. Breastfeed Med,5:283-288.
[7]. Payne, J.L. and Maguire, J. (2018) Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol, 52: 165-180.
[8]. Grzeskowiak, L., et al. (2022) Perinatal antidepressant use and breastfeeding outcomes: Findings from the Norwegian Mother, Father and Child Cohort Study. Acta Obstet Gynecol Scand, 101: 344-354.
[9]. Venkatesh, K., et al. (2017) Impact of antidepressant treatment during pregnancy on obstetric outcomes among women previously treated for depression: An observational cohort study. J Perinatol, 37: 1003-1009.
[10]. Hendrick, V., et al. (2001) Venlafaxine and breast-feeding. Am J Psychiatry.
[11]. Uguz, F. (2021) A new safety scoring system for the use of psychotropic drugs during lactation. Am J Ther, 28: e118-e126.
[12]. Desaunay, P., et al. (2023) Benefits and risks of antidepressants during pregnancy: a systematic review of a meta-analysis. Pediatric drugs, 25(3): 247-265.
[13]. Berle, J.Ø., et al. (2004) Breastfeeding during maternal antidepressant treatment with serotonin reuptake inhibitors: infant exposure, clinical symptoms, and cytochrome p450 genotypes. J Clin Psychiatry. 65(9): 1228-1234.
[14]. Pons, G., Rey, E. and Matheson, I. (1994) Excretion of psychoactive drugs into breast milk. Pharmacokinetic principles and recommendations. Clin Pharmacokinet. 27(4): 270-289.
[15]. Kieviet, N., et al. (2015) Risk factors for poor neonatal adaptation after exposure to antidepressants in utero. Acta Paediatr, 104: 384-391.
[16]. Kronenfeld, N., et al. (2018) Chronic use of psychotropic medications in breastfeeding women: Is it safe? PLoS One, 13: e0197196.









