1. Introduction
1.1. Background
Adult hippocampal neurogenesis (AHN) refers to the process by which neural stem cells (NSCs) can proliferate and differentiate into functionally integrated neurons in the adult mammalian brain, particularly in the dentate gyrus (DG) region of the hippocampus [1]. This process is important for understanding brain plasticity and the pathogenesis of certain neuropsychiatric disorders such as depression, Alzheimer's disease, etc [2].
Studies have shown that adult hippocampal neurogenesis is closely related to cognitive function. Newly generated neurons play an important role in memory formation, learning, and cognitive flexibility. They may enhance brain plasticity by participating in the reorganization and renewal of neural networks [3].
Neuropsychiatric abnormalities in the adult hippocampus are associated with a variety of neuropsychiatric disorders. For example, a decrease in hippocampal volume and neurogenesis was observed in patients with depression; In patients with Alzheimer's disease, severe inhibition of neurogenesis is found [4].
A crucial subcortical area of the hypothalamus, the supramammillary nucleus (SuM) is highly receptive to pro-neurogenic stimuli and sends many projections to the DG. When mice are exposed to a novel environment, SuM neurons show higher firing frequency, calcium dynamics, and c-Fos expression. Crucially, ablation of SuM neurons eliminates the effects of environmental novelty (EN)-induced augmentation of AHN, indicating that SuM neurons are necessary for this process. These results suggest the intriguing prospect that activating the SuM could resemble EN-induced AHN amplification.
The number of behaviorally relevant ABNs with improved developmental features increases when SuM neurons are patterned optogenetically stimulated at a frequency that mimics their firing rate in the novel environment.[5]. A tiny population of time-stamped SuM-enhanced ABNs can be acutely chemogenetic activated to boost memory function and decrease anxiety-like behavior. Even a little increase in the activity of SuM-enhanced ABNs can have a major positive behavioral impact.
1.2. Previous research
Using two different AD mice models, 5×FAD and 3×Tg-AD, researchers tested this innovative AHN-enhancing method in deteriorated AD brains to see if it might restore AHN and achieve functional recovery [1].
Researchers discovered that the quantity and developmental characteristics of ABNs are restored in AD mice at different disease stages (from early to late) with programmed optogenetic activation of SuM. Significantly, memory can be restored and anxiety/depression-like behaviors can be decreased in AD mice by acutely chemogenetic activation of a limited population of SuM-enhanced ABNs. On the other hand, behavioral impairments in AD mice cannot be restored by SuM stimulation alone or by acute activation of ABNs without SuM alteration [1]. They carried out quantitative phosphoproteomics of the hippocampus after acute chemogenetic activation of SuM-enhanced ABNs in order to investigate ABN-activity-dependent processes. Their investigations showed that acute activation of SuM-enhanced ABNs activated the canonical pathways linked to synaptic plasticity and microglia plaque phagocytosis [1].
The findings show that in two different AD mice models, 5×FAD and 3×Tg-AD, patterned optogenetic activation of the hypothalamic supramammillary nucleus (SuM) increases AHN. Interestingly, these AD mice's memory and emotional deficiencies are restored when SuM-enhanced adult-born neurons (ABNs) are chemogenetic activated. In contrast, behavioral impairments cannot be restored by SuM stimulation alone or by activating ABNs without modifying SuM (see Figure 1). Moreover, after acute chemogenetic activation of SuM-enhanced (as opposed to control) ABNs, quantitative phosphoproteomics analyses show activation of the canonical pathways associated with synaptic plasticity and microglia phagocytosis of plaques. The research demonstrates the activity-dependent role of SuM-enhanced ABNs in modifying impairments associated with AD and provides insights into the signaling pathways that are triggered by the activation of SuM-enhanced ABNs [1].
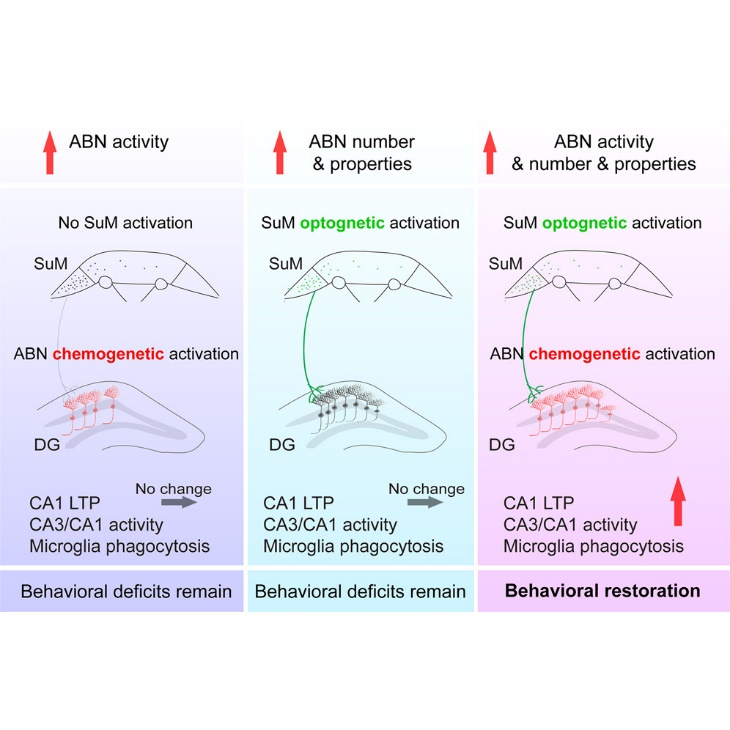
Figure 1: Graphocal abstract of previous research [1]
1.3. Highlight
Generally, in AD mice who received SuM-enhanced ABNs activation, researchers found that microglia were activated and had an increased ability to engulf Abeta.
It’s already known that both activation of SuM-enhanced ABNs and activation of microglia were associated with cognitive and emotional improvements in AD mice. But who directly led to the improvements remains unclear? Besides, we still don’t know the relationship between ABNs and microglia.
In this work, regarding the direct factors of improvements in cognition and emotion, we suppose that is caused by both SuM-enhanced ABNs activation and microglia activation. Therefore, the second question emerges: who activate microglia? From our perspective, both Abeta and SuM-enhanced ABNs activation can stimulate microglia. After solving these two questions above, we wonder whether memory and cognitive enhancement lasts long, so we design a third experiment to figure out the question.
2. Experiments
2.1. Explore the mechanism of cognitive and emotional function enhancement
2.1.1. Hypothesis
In previous research, the activation of AHN by SuM results in an enhancement of cognitive and emotional functioning in mice. But the morphology and behaviors of microglia were both changed in their experiments. It’s unkown whether microglia behaviors would influence emotional and cognitive abilities, the exact association between microglia and ABNs is not clear yet. Therefore, based on the previous experimental results, our hypothesis is that ABNs are the underlying cause of changes in cognitive ability and mood. The behaviour of microglia may contribute indirectly.
2.1.2. Method
To study ABNs and microglia separately. We devise two approaches where they can individually block ABNs and microglial cells separately.
The survival of microglia relies on colony-stimulating factor 1 receptor (CSF1R) signaling. Efficacy in inducing microglial elimination with CSF1R inhibitors (99%). CSF1R inhibitor PLX3397 is an FDA-approved drug for the treatment of glioblastoma. Some clinical data suggest that the drug has a high efficacy in the depletion of microglia in the human brain [6]. We added this to the feed of the model mice for microglial elimination.
In order to remove ABNs completely, we revert to using CRE proteins system (see Figure 2). A neural stem cell-exclusive NSC promoter was used, and then an shRNA sequence was added after the loxp sequence [7]. We hope that by means of shRNA interference, the shRNA will bind to the mRNA of cycE after the addition of tamoxifen. As a result, the neural stem cell cycle will be abnormal and ABNs will be completely blocked.
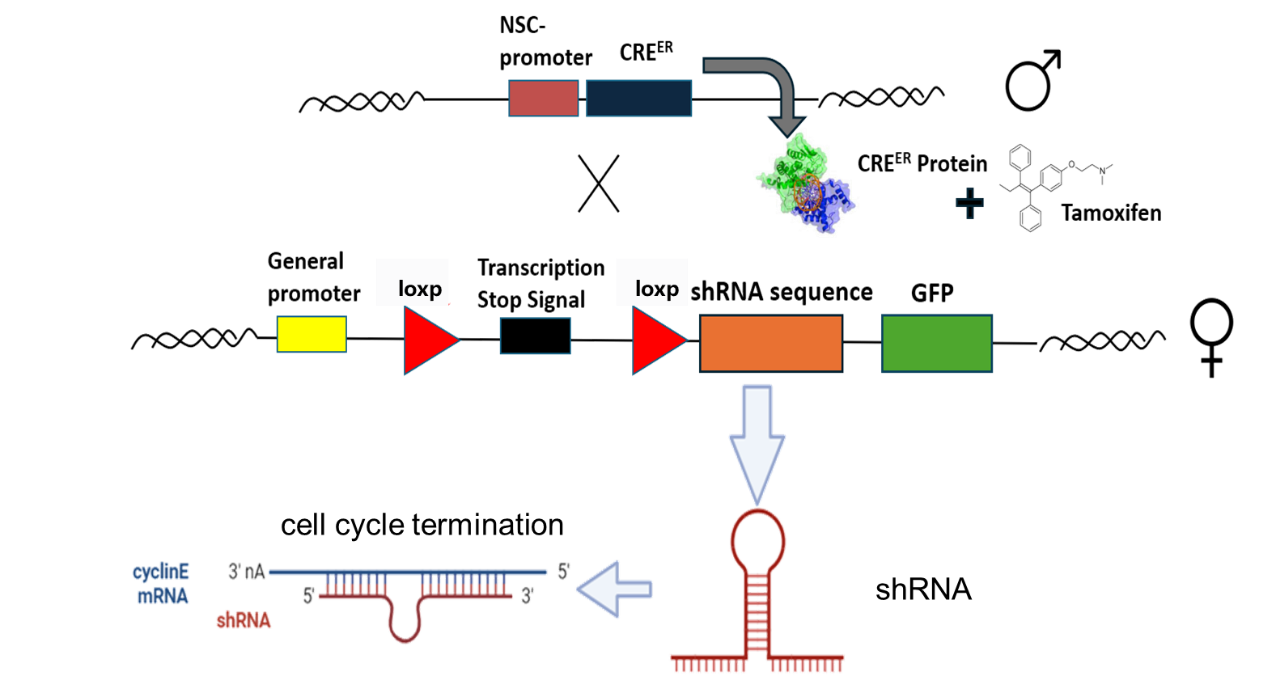
Figure 2: CRE system to remove ABNs
The FAD mouse was used as a model. We designed a total of four groups, blocking ABNs, blocking microglia, and blocking nothing, stimulating both SuM and ABNs as positive control, and doing nothing to the model as negative control (see Figure 3).
Finally, we will verify whether the cognitive abilities and emotions of the mice are altered by behavioral tests, which include Novel Object Recognition (NOR), Novel Location Recognition (NLR), Morris Water Maze (MWM) and Contextual Fear Conditioning (CFC) test.
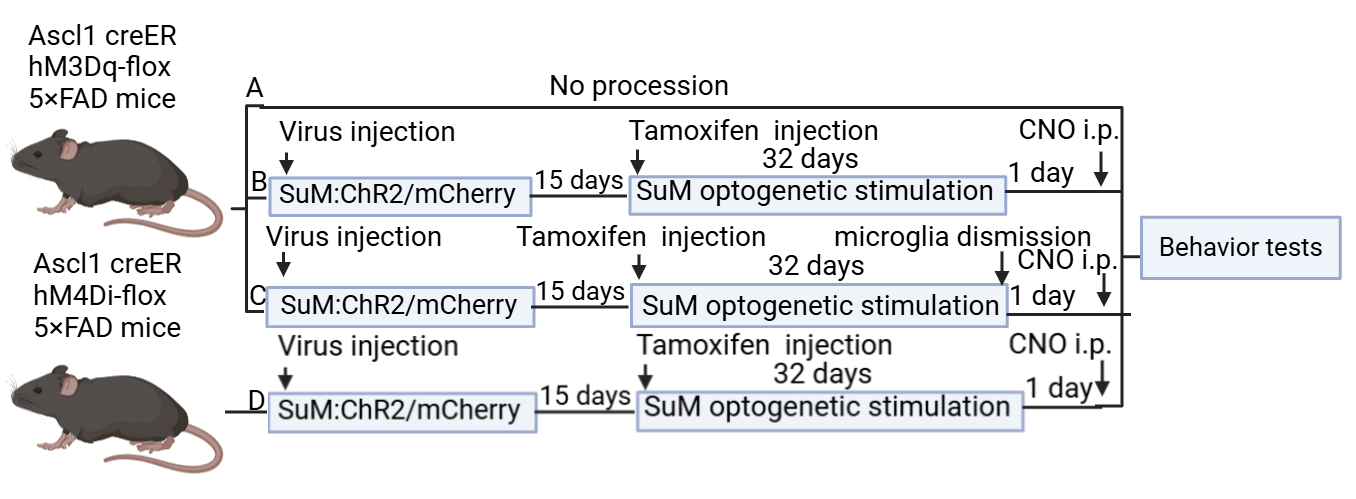
Figure 3: Experiment 1 design
2.1.3. Expected result
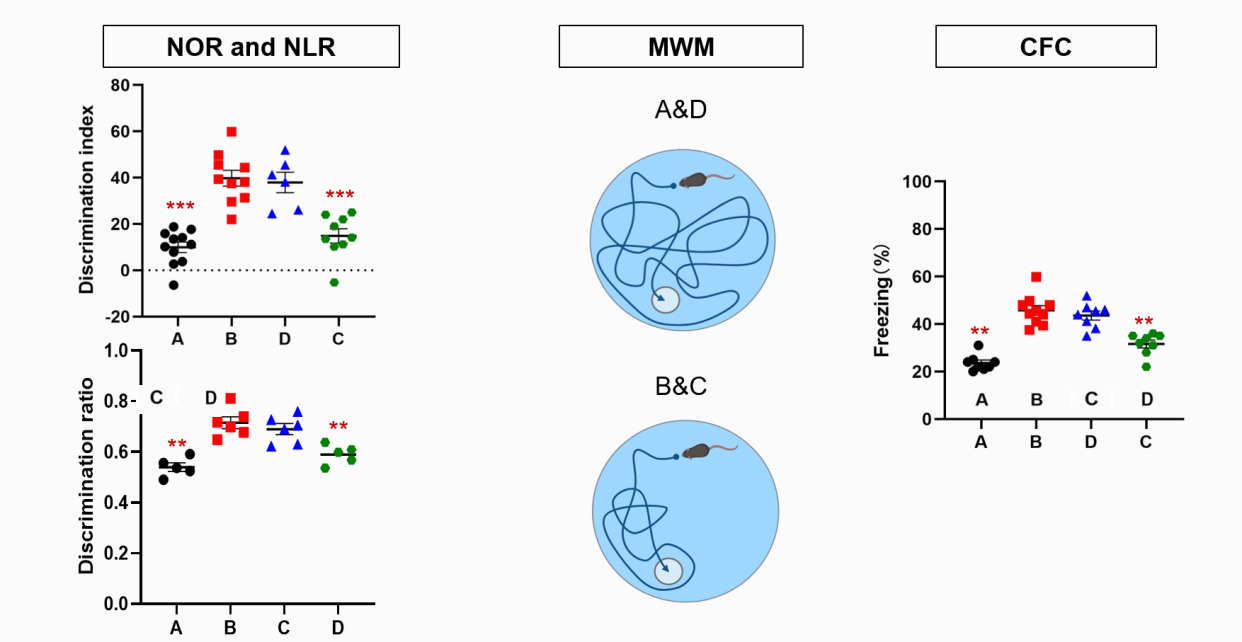
Figure 4: Experiment 1 expected result
As shown in Figure 4, in the NPR experiment, Groups B and C performed better because they have ABNs. Group D performs worse but better than Group A. Because we predict that microglia have something to do with Cognition and emotion, like indirectly impact or something. And in MWM test group B and C perform better in Memorizing the location of the platform.
2.2. The activition of microglia
2.2.1. Hypothesis
In the previous research, we already knew that ABN can activate microglia and enhance their phagocytic activity [1]. However, in the pathological mechanism of AD, on the one hand, microglia can be activated by Aβ plaques and exert their phagocytic activity, clearing Aβ and damaged cells [8]; Microglia can also induce the clearance of Aβ by releasing proteases that degrade Aβ, such as insulin degrading enzyme, plasminogen [9]. Therefore, we want to investigate whether microglia are activated by ABN or by Aβ plaques in the context of SuM activation.
So, we made the following hypothesis:
Hypothesis 1: Microglia is only activated by A β and is not related to the activation of SuM. Hypothesis 2; Microglia are activated by both Aβ and SuM, and the degree of simultaneous activation of both is higher than that of activation by Aβ alone.
2.2.2. Method
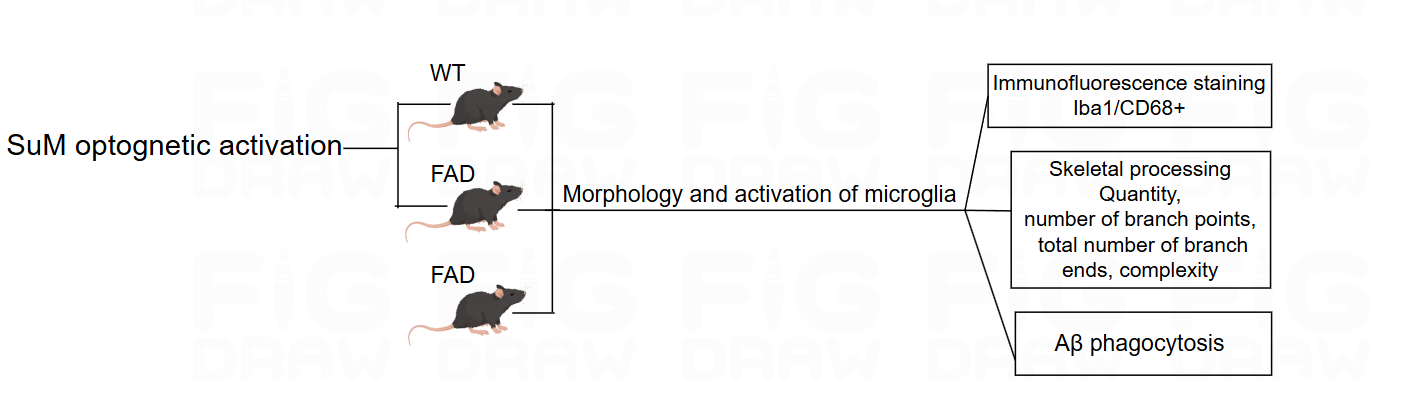
Figure 5: Experiment 2 design
The experimental design consists of four groups of mice, Group A and D are wild-type mice, while Group BC and are FAD mice (see Figure 5). Group AB mice activate SuM using optogenetic methods, while Group CD remains inactive. Afterwards, the activation status of microglia and their ability to phagocytize Aβ plaques are analysed. The analysis content is divided into three parts. Firstly, immunofluorescence staining is used. Iba1 is a marker for microglia, CD68 is a marker for microglial activation and phagocytosis, and photos of microglia are taken under confocal fluorescence microscopy [10]; the activation of microglia is mainly reflected in their morphological changes, from a highly branched resting state to an enlarged cell body in an amoeboid activated state [11]. Therefore, we will use ImageJ software and NeuroJ plugin to analyze the Quantity, number of branch points, total number of branch ends, complexity of microglia to determine their activation status. Finally, we will test the ability of microglia to phagocytize Aβ plaques.
2.2.3. Expected result
Table 1: Experiment 2 expected result
Expected result | 1 | 2 | 3 |
Activation of microglia | WT × | WT √ FAD√ | WT √ FAD√ |
Activation and phagocytosis | BC two groups comparable | Group B stronger than those of Group C | |
Conclusion | Only activated by Aβ | Both Aβ and SuM | Both Aβ and SuM Activation effect enhanced |
According to Table 1, if the microglia in the WT group are not activated after activating SuM, it indicates that only A β activates the microglia. If both WT and FAD groups activate microglia after activating SuM, it indicates that microglia are activated by both Aβ and SuM. Group B has SuM activation, while Group C does not. If the activation degree and phagocytic ability of microglia in FAD mice in Group BC are the same, it indicates that the activation ability of Aβ plaques and SuM on microglia is similar. If the activity and phagocytic ability of microglia activated by SuM in group B are stronger than those in group C, it indicates that microglia are not only activated by both, but also have a stronger ability to be activated by both than by Aβ alone.
2.3. The long-term effect of SuM modified ABNs on memory and emotion function of AD mice
At last, we want to investigate the long-term effect of SuM modified ABNs on memory and emotion function of AD mice. It’s known that SuM optogenetic activation then ABN chemogenetic activation on the 5×FAD mice will activate the microglia to be amoebic so that enhance the plaque phagocytosis, finally improve cognitive and emotional ability [12]. Then the question comes out that is it a long term improvement?
2.3.1. Hypothesis
To answer this question, we put forward three hypothese. First, the function of SuM modified ABNs on AD mice is temporary. Second, the function of SuM modified ABNs on AD mice has a long-term effect. Third, the function of SuM modified ABNs on AD mice decreases over time.
2.3.2. Method
To validate these hypotheses,we developed the following experiment protocols (see Figure 6):first,dividing severe Ascl1 creER hM3Dq-flox 5×FAD(AM3AD) mice to A, B, C three groups, ChR2 virus injection to SuM on half of each group,mCherry virus injection to SuM on other mice of each group.15 days later, tamoxifen injection to all groups to turn on the hM3Dq gene. Then, SuM optogenetic stimulation is carried out for 32 days. To avoid acute effect from SuM stimulation,1 day later, CNO i.p. are given to all groups for chemogenetic activation of SuM-modified or control ABNs [13]. The three groups of mice A, B, and C undergo behavioral tests and Imaris imaging analysis after 30-60 min, 10 days later, and 30 days later, respectively.
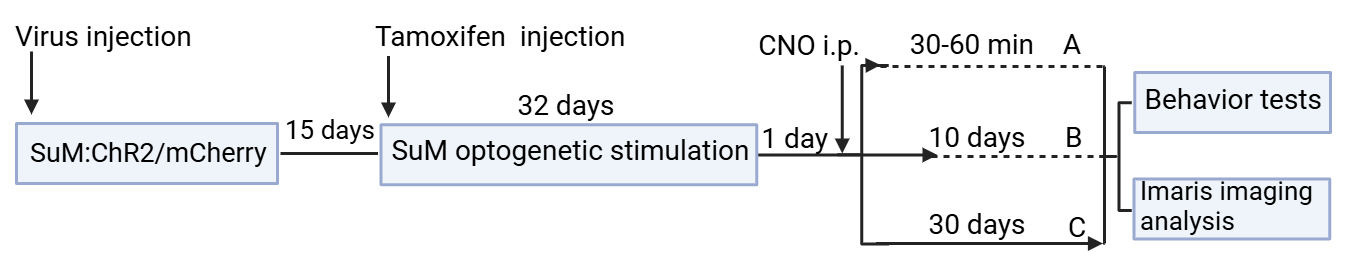
Figure 6: Experiment 3 design
2.3.3. Expected result
Table 2: Experiment 3 expected result
Result | 1 | 2 | 3 |
Cognitive and affective ability | A=B=C | A>B>C | A>B=C or A=B>C |
Microglia phagocytosis ability | A=B=C | A>B>C | A>B=C or A=B>C |
Conclusion | Hypothesis 1 or 2 | Hypothesis 3 | Hypothesis 1 |
According to Table 2, by comparing the cognitive and emotional abilities and microglia phagocytosis improvement of ChR2 AM3AD mice and mCherry AM3AD mice in the three groups A, B, and C,if A=B=C, there are two possibilities: The function of SuM modified ABNs on AD mice has a long term effect,or one month is too short.hypothesis 1 or 2 is right,if A>B>C, this means the function of SuM modified ABNs on AD mice decreases over time,hypothesis 3 is right,if A>B=C or A=B>C, this means the function of SuM modified ABNs on AD mice is temporary,hypothesis 1 is right. If result 1 appears, further experiment will delay ABN activation to validate whether the function of SuM modified ABNs on AD mice is permanent.
3. Conclusion
3.1. Research implications
It has been confirmed that the activation of ABN and microglia can directly improve the learning memory and emotional regulation of AD mice. But the detailed mechanism of it is not clear.
Our research will figure it out how ABN and microglia independently and jointly regulate cognitive and emotional function, this will help to discover new therapeutic targets. For example, the study of drugs that can activate ABNs or microglia, rather than directly using antibodies bound to Abeta to activate immune cell phagocytosis.
The mechanism of microglia activition will also be further clarified by this research, which is important for understnding the role of microglia in Alzheimer's disease.
Finally, our research will investigate the long-term effect of SuM modified ABNs on memory and emotion function of AD mice, which suggests that early intervention in Alzheimer's disease is possible if it does exist.
3.2. Limitation
This study has certain drawbacks. Initially, we employed 5×FAD, a single AD mouse model that overexpresses the disease genes. It does not, however, fully reproduce the diseases of AD in humans. To expand our findings to a more physiological context for AD disorders, we plan to investigate knockin mice models of AD in the future. Secondly, our investigation does not go into detail about molecular investigations, such as phosphoproteomics.
3.3. Research prospect
There are also some further researches that we hope to deal with in the future. We want to explore whether the activation of microglia augments the function of ABNs. And we also expect to figure out the specific effect of ABN maturity quantity activity through analyzing Golgi staining, the density of newly generated neuronal dendritic spines/number of synaptic branches, which can also be used as indicators to determine whether the activation of microglia can enhance the function of ABNs.
References
[1]. Li, Y.D., Luo, Y.J., Song, J. (2023). Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer’s disease. Cell Stem Cell, 30, 415–432.
[2]. Li, Y.D., Luo, Y.J., Song, J. (2022). Hypothalamic modulation of adult hippocampal neurogenesis in mice confers activity-dependent regulation of memory and anxiety-like behavior. Nature neuroscience,25,630-645.
[3]. Moreno-Jime´nez, E.P., Flor-Garcı´a, M., Terreros-Roncal, J., Ra´bano, A., Cafini, F., Pallas-Bazarra, N., A ´vila, J., and Llorens-Martı´n, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560.
[4]. Tobin, M.K., Musaraca, K., Disouky, A., Shetti, A., Bheri, A., Honer, W.G., Kim, N., Dawe, R.J., Bennett, D.A., Arfanakis, K., and Lazarov, O. (2019). Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24, 974–982.e3.
[5]. Zhang, X., Wei, X., Mei, Y., Wang, D., Wang, J., Zhang, Y., Li, X., Gu, Y., Peng, G., and Sun, B. (2021). Modulating adult neurogenesis affects syn aptic plasticity and cognitive functions in mouse models of Alzheimer’s disease. Stem Cell Rep. 16, 3005–3019.
[6]. Wang, W.B., Li, Y.Z., Ma, F.L., Zhong, L., et al. (2023). Microglial repopulation reverses cognitive and synaptic deficits in an Alzheimer’s disease model by restoring BDNF signaling. Brain, Behavior, and Immunity, 113, 275-288.
[7]. Zhu, H., Aryal, D.K., Olsen, R.H., Urban, D.J., Swearingen, A., Forbes, S., Roth, B.L., and Hochgeschwender, U. (2016). Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54, 439–446.
[8]. Sarlus, H., and Heneka, M.T. (2017). Microglia in Alzheimer’s disease. J. Clin. Invest. 127, 3240–3249.
[9]. Franco-Bocanegra, D.K., McAuley, C., Nicoll, J.A.R., and Boche, D. (2019). Molecular mechanisms of microglial motility: changes in ageing and Alzheimer’s disease. Cells 8, 639.
[10]. Gonza´lez Ibanez, F., Picard, K., Bordeleau, M., Sharma, K., Bisht, K., and Tremblay, M.E `. (2019). Immunofluorescence staining using IBA1 and TMEM119 for microglial density, morphology, and peripheral myeloid cell infiltration analysis in mouse brain. J. Vis. Exp.
[11]. Leng F, Edison P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol, 17(3), 157-172.
[12]. Hu, J., Chen, Q., Zhu, H.R. (2023). Microglial Piezo1 senses Aβ fibril stiffness to restrict Alzheimer's disease. Neuron, 111(1), 15- 29.
[13]. Tong, L., Han, S.S., Xue, Y. (2023). Single cell in vivo optogenetic stimulation by two-photon excitation fluorescence transfer. iScience,107857.
Cite this article
Liu,X. (2025). The Mechanism of Activating SuM Modified ABN to Improve Memory and Emotional Function in AD. Theoretical and Natural Science,115,1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, Y.D., Luo, Y.J., Song, J. (2023). Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer’s disease. Cell Stem Cell, 30, 415–432.
[2]. Li, Y.D., Luo, Y.J., Song, J. (2022). Hypothalamic modulation of adult hippocampal neurogenesis in mice confers activity-dependent regulation of memory and anxiety-like behavior. Nature neuroscience,25,630-645.
[3]. Moreno-Jime´nez, E.P., Flor-Garcı´a, M., Terreros-Roncal, J., Ra´bano, A., Cafini, F., Pallas-Bazarra, N., A ´vila, J., and Llorens-Martı´n, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560.
[4]. Tobin, M.K., Musaraca, K., Disouky, A., Shetti, A., Bheri, A., Honer, W.G., Kim, N., Dawe, R.J., Bennett, D.A., Arfanakis, K., and Lazarov, O. (2019). Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24, 974–982.e3.
[5]. Zhang, X., Wei, X., Mei, Y., Wang, D., Wang, J., Zhang, Y., Li, X., Gu, Y., Peng, G., and Sun, B. (2021). Modulating adult neurogenesis affects syn aptic plasticity and cognitive functions in mouse models of Alzheimer’s disease. Stem Cell Rep. 16, 3005–3019.
[6]. Wang, W.B., Li, Y.Z., Ma, F.L., Zhong, L., et al. (2023). Microglial repopulation reverses cognitive and synaptic deficits in an Alzheimer’s disease model by restoring BDNF signaling. Brain, Behavior, and Immunity, 113, 275-288.
[7]. Zhu, H., Aryal, D.K., Olsen, R.H., Urban, D.J., Swearingen, A., Forbes, S., Roth, B.L., and Hochgeschwender, U. (2016). Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54, 439–446.
[8]. Sarlus, H., and Heneka, M.T. (2017). Microglia in Alzheimer’s disease. J. Clin. Invest. 127, 3240–3249.
[9]. Franco-Bocanegra, D.K., McAuley, C., Nicoll, J.A.R., and Boche, D. (2019). Molecular mechanisms of microglial motility: changes in ageing and Alzheimer’s disease. Cells 8, 639.
[10]. Gonza´lez Ibanez, F., Picard, K., Bordeleau, M., Sharma, K., Bisht, K., and Tremblay, M.E `. (2019). Immunofluorescence staining using IBA1 and TMEM119 for microglial density, morphology, and peripheral myeloid cell infiltration analysis in mouse brain. J. Vis. Exp.
[11]. Leng F, Edison P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol, 17(3), 157-172.
[12]. Hu, J., Chen, Q., Zhu, H.R. (2023). Microglial Piezo1 senses Aβ fibril stiffness to restrict Alzheimer's disease. Neuron, 111(1), 15- 29.
[13]. Tong, L., Han, S.S., Xue, Y. (2023). Single cell in vivo optogenetic stimulation by two-photon excitation fluorescence transfer. iScience,107857.









