1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized behaviorally by progressive memory loss and cognitive decline. Pathologically, AD is marked by extracellular amyloid-beta (Aβ) plaques and intracellular neurofibrillary tangles. These pathological changes most severely impact hippocampus, a brain area essential for memory formation and consolidation [reviewed by 1]. Among hippocampal subfields, the dentate gyrus (DG) shows evidence of functional impairment during the stages of generating, differentiating, and maturing new neurons in aging AD patients [2]. This process by which new neurons are produced in adult brains is referred to as adult hippocampal neurogenesis (AHN) [reviewed by 3].
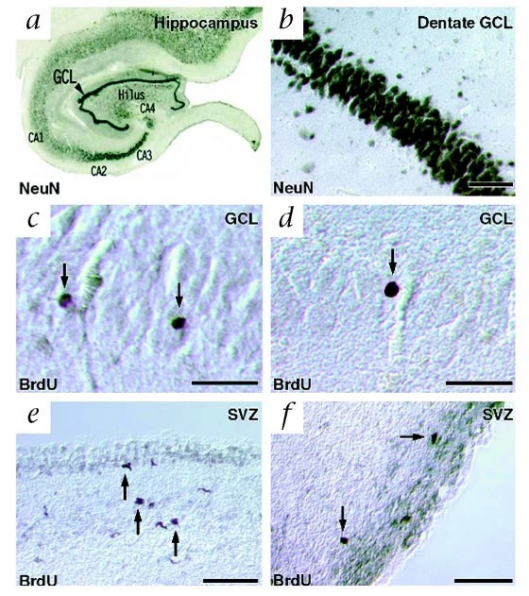
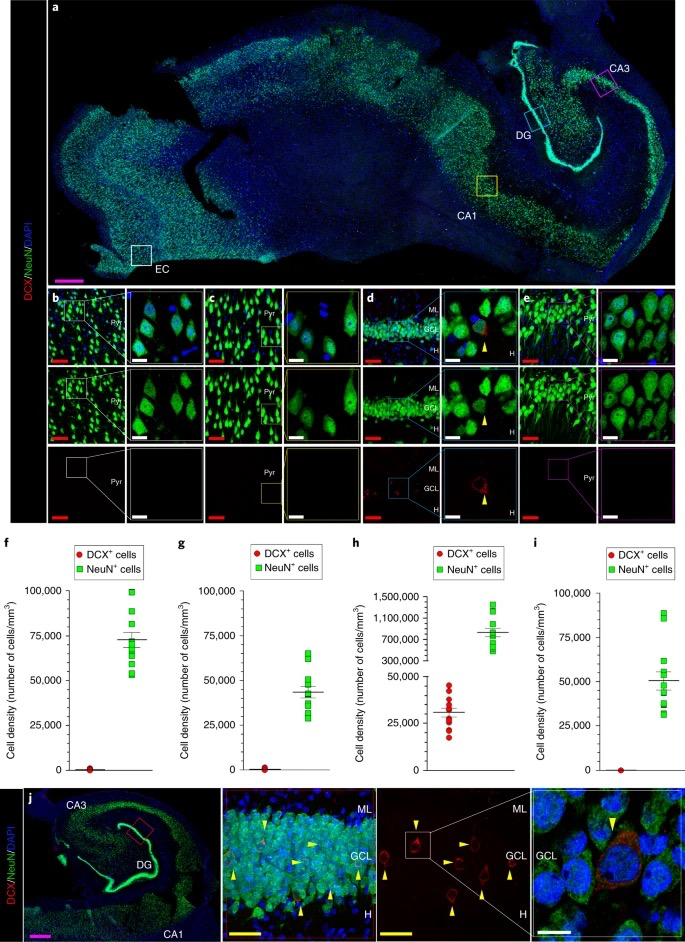
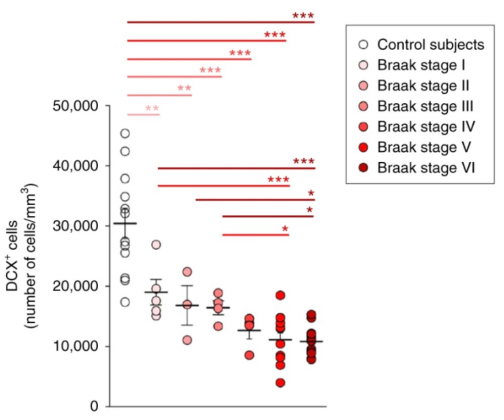
In fact, for a very long time, neurogenesis has been considered not to occur after birth [4]. Not until 1960s, undifferentiated neurons found in the DG of mammalian hippocampus suggested delayed neurogenesis in adulthood [5]. AHN thereafter has been studied with animal models, whereas limited methods could be used to study neurogenesis in living human. In 1998, Eriksson et al. first found evidence of new neurons differentiated from progenitor cells, as labeled by bromodeoxyuridine (BrdU), a marker of cell division, in human postmortem tissue sample (Figure 1a-d) [6]. Despite the hot debate raised after, that the number of new neurons may be significantly fewer than initial thought [7], Moreno-Jimenez et al. largely improved on the tissue processing method and acquired abundant data from human postmortem tissue sample, suggesting the occurrence of AHN in human at all ages (Figure 1e). Nevertheless, the most noteworthy observation from their study was the statistically significant amount of AHN impairment found in AD sample compare to healthy controls (Figure 1f). Specifically, they observed a decline in the expression of protein markers associated with different stages of neuronal maturation as AD advanced [2]. The potential connection between AHN and AD found in human introduced novel mechanisms contributing to understand the disease and develop therapeutics.
|
|
Figure 1: Impaired adult hippocampal neurogenesis in the dentate gyrus of Alzheimer's disease patients. a-b) Immunostaining shows NeuN-expressing neurons in the hippocampus and the dentate gyrus. c-d) BrdU-labeled nuclei, indicative of new cells, are observed in the granule cell layer of the dentate gyrus [6]. e) Density of DCX- and NeuN-expressing cells in the dentate gyrus of thirteen neurologically healthy human aged 43 to 87. f) Quantification shows a significant decrease in DCX-expressing cells as Alzheimer’s disease progresses [2] | |
Later studies improved on the tissue processing method and further suggested neurogenesis impairment in AD patients [8, 9]. However, the reduction in AHN remains a hypothesis due to the limitation to progressively observe each maturation stages of new neurons during the neurogenesis processes in human sample. Thus, the causal relationship between the decreased AHN and AD events still needs further investigation. In this review, I will discuss the current knowledge regarding the state of AHN in AD, specifically highlighting how AHN is altered in AD samples. I will also explore underlying mechanisms that may contribute to AHN dysfunction and examine emerging therapeutic strategies that aim to target neurogenesis as a treatment for AD. Lastly, I will further discuss future methodologies and directions to study AHN in AD.
2. Alterations of adult hippocampal neurogenesis in Alzheimer's disease
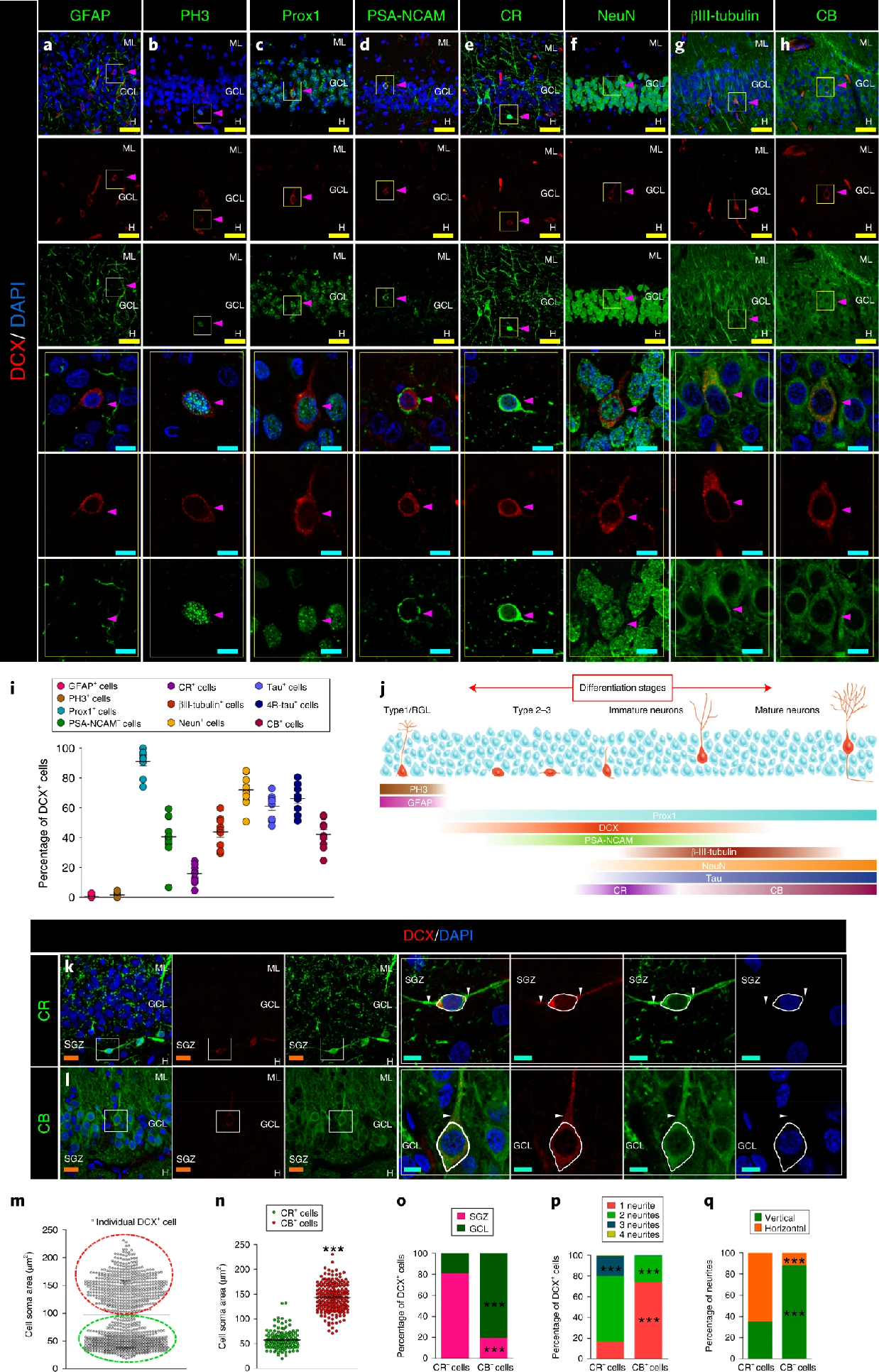
In healthy individuals, AHN initiates with quiescent neural stem cells (NSCs) located predominantly in the subgranular zone (SGZ) of the DG within the hippocampus. Upon activation, NSCs differentiate into neural progenitor cells (NPCs), which proliferate and further develop to immature neurons. Then, these immature neurons extend axons and dendrites, establishing synaptic connections and integrating into hippocampal circuits (Figure 2), thereby contributing to cognitive functions like learning and memory [reviewed by 3]. In contrast, in AD patients, while the number of NSCs in the DG is similar to healthy individuals, these NSCs tend to remain in a quiescent state rather than activated [8]. Moreover, immunohistochemical analysis of postmortem AD tissue reveals decreased expressions of key proteins involved in neuronal migration, including Doublecortin (DCX) and Polysialylated Neural Cell Adhesion Molecule (PSA-NCAM), suggesting a reduced transition from NSCs to NPCs in AD compared to healthy controls [2, 8]. Reductions in expressions of BrdU, prospero-related homeobox 1 (Prox1), βIII tubulin, NeuN, calretinin (CR), and calbindin (CB) indicate impairments in NPC proliferation and in the maturation of immature neurons [1-2, 8]. Collectively, these impairments across all stages of AHN suggest a potential link between disrupted neurogenesis and the pathophysiology of AD.
|
Figure 2: Maturation model of adult hippocampal neurogenesis in humans. Radial glia-like stem cell (Type1/RGL) transition into progenitor cells (Type 2-3) and immature neurons before reaching full maturity. The progression is marked by the expression of key proteins: Prox1 (Prospero-related homeobox 1), DCX (Doublecortin); PSA-NCAM (Polysialylated neural cell adhesion molecule); β-III-tubulin; NeuN; Tau; CR (Calretinin); and CB (Calbindin) [2] |
Notably, changes in AHN have been identified in the early phases of AD patients, even before the buildup of Aβ plaques and neurofibrillary tangles [2]. This phenomenon is also evident in animal models of AD. In transgenic mice expressing mutations in presenilin 1 (PSEN1) and amyloid precursor protein (APP), which model genetically inherited familial AD (FAD), the production and survival of new neurons were already compromised by three months of age, long before plaque deposition or memory deficits appeared at nine months [10]. In senescence-accelerated OXYS rats, which model sporadic AD (SAD) of non-genetic origin, delayed hippocampal development and reduced neurogenesis were observed as early as postnatal day 10, contributing to developing AD-like pathology later in life [11]. These studies suggest that alterations in AHN are not merely coincidental with the progression of AD but may actively contribute to its onset. Promisingly, AHN alterations might serve as an early diagnostic marker for AD, offering a window for early intervention before the disease progresses.
Stem cell transplantation therefore offers a possible therapeutic approach for AD by artificially enhancing AHN, potentially restoring healthy NSC levels in AD patients at an early stage. From an existing study, transplanted human NSCs derived from fetal telencephalon into an NSE/APPsw (neuron-specific enolase promoter-controlled APPsw-expressing) transgenic AD mouse model were able to migrate and differentiate into mature neurons and glial cells, leading to a reduction of Aβ level, tau phosphorylation, and neuroinflammation [12]. Further advancements in this area, such as pharmacologically inhibiting asparaginyl endopeptidase (AEP), have improved the brain microenvironment for NSC survival, significantly enhancing therapeutic efficacy [13]. Although challenges remain, such as immunological rejection and ensuring long-term efficacy in humans, NSC transplantation holds great potential and warrants further exploration.
3. Disrupted signaling pathways in AHN and AD
Several molecular signaling pathways involve in regulating AHN, including Wnt, phosphoinositide 3-kinase (PI3K)/ Akt, and brain-derived neurotrophic factor (BDNF) signaling pathways. These pathways are also disrupted in AD, suggesting that their impairment may underlie altered AHN and presenting a potential avenue for therapeutic intervention to restore neurogenesis.
3.1. Wnt signaling pathway
The Wnt signaling network consists of canonical and noncanonical pathways, each with distinct roles in AHN. The canonical Wnt pathway, triggers by Wnt ligands binding to membrane receptors, stabilizes β-catenin, allowing its nuclear translocation to promote the expression of genes related to cell proliferation, survival, and differentiation. In contrast, the noncanonical pathway regulates cell polarity and migration [reviewed by 14]. Nonetheless, in the context of AD, Aβ plaques interrupt β-catenin signaling downstream in the canonical Wnt pathway, contributing to disrupted neurogenesis in AD progenitor cells [15]. Upstream, the low-density lipoprotein receptor-related protein 6 (LRP6), which stabilizes β-catenin, is also downregulated in AD, directly resulting in dendritic spine loss and memory decline. The dysfunction of the LRP6-mediated Wnt signaling pathway further exacerbates Aβ plaque formation, thereby accelerating neurodegeneration [16]. Therefore, elevating the level of β-catenin or LRP6 may be a prospective therapeutic strategy for AD. A recent study has already shown that modulating LRP6 in the Wnt signaling pathway within brain endothelial cells can help restore blood–brain barrier, which has been compromised by amyloid-β accumulation in AD mouse models [17]. In particular, a variant of LRP6, LRP6-Val, is related to greater synapse degeneration [18], suggesting a potential therapeutic target for future studies. Additionally, sirtuin 3 (SIRT3), a deacetylase involved in preventing age-related declines in neurogenesis [19], has been shown to promote neurogenesis in AD mice through modulation of the Wnt signaling pathway [20]. Overall, these findings highlight the Wnt signaling pathway as an important area for understanding AD pathology and developing targeted treatments, warranting further research.
3.2. PI3K/Akt signaling pathway
The PI3K/Akt pathway supports neuronal and NSC survival, proliferation, differentiation, and maturation, making it a key target for promoting AHN. Specifically, growth factors activate the PI3K catalytic subunit, which in turn activates Akt to integrate with downstream proteins. For instance, Akt can phosphorylate glycogen synthase kinase-3β (GSK-3β), inhibiting its pro-apoptotic function, thus allowing NSCs to survive and differentiate into neurons, astrocytes, and oligodendrocytes. In AD, however, Aβ oligomers inhibit the PI3K/Akt pathway, increasing GSK-3β activity, which promotes apoptosis while contributes to Tau hyperphosphorylation [reviewed by 21]. Thus, to restore the PI3K/Akt pathway as a therapeutic approach, one study suggested the use of β-amyrin, a natural compound that reactivates the PI3K/Akt pathway in the presence of Aβ, as tested in Aβ-injected AD mouse models [22]. Yet, further research is needed to test its efficacy.
3.3. BDNF signaling pathway
The BDNF pathway is essential for maintaining synaptic plasticity and neuronal survival, including newly generated neurons from AHN. The precursor form, proBDNF, binds to the p75 neurotrophin receptor (p75NTR) to promote long-term depression and apoptosis; mature BDNF, after cleavage, binds to the TrkB receptor to activate pathways involved in long-term potentiation and survival [reviewed by 23]. In AD, both BDNF and proBDNF levels are largely reduced, correlating with disease progression and cognitive decline, particularly episodic memory loss [23, 24]. Aβ pathology also lowers the level of phosphorylated CREB protein, thus inhibiting CREB-regulated BDNF transcription and consequently reducing neurotrophic support [reviewed by 23]. Promisingly, in a recent study, Ginsenoside RK3 (RK3), a substance found in traditional Chinese herbs, has been shown to improve cognition and learning in transgenic AD mice by activating the CREB/BDNF pathway to enhance hippocampal neurogenesis and synaptogenesis [25]. Similarly, curcumin, a bioactive substance from Asia, has been shown to enhance CREB activity and thereby increase BDNF levels in AD mice. Curcumin also reduces GSK3β activity by activating the PI3K/Akt pathway and enhances the Wnt/β-catenin pathway [26]. Together, these findings underscore the therapeutic potential of modulating neurogenic pathways like CREB/BDNF.
4. Synaptic integration of new neurons in Alzheimer's disease
In 2002, van Praag et al. indicated that newly generated neurons from AHN functionally integrate into the hippocampal circuitry [27]. Using a retroviral vector expressing fluorescent proteins in the DG and electrophysiology, they recorded that these new neurons receive input from the perforant pathway and share intrinsic characteristics, such as resting potential, input resistance, and firing thresholds, with mature neurons. Notably, the new neurons display distinct synaptic behavior, indicating unique presynaptic release properties [27]. In AD, however, proteins involved in neuronal signaling and synaptic integration, such as PSA-NCAM, βIII tubulin, CR, and CB, significantly decline [2], suggesting that immature neurons in AD may struggle to integrate into functional hippocampal circuits, potentially contributing to memory deficits.
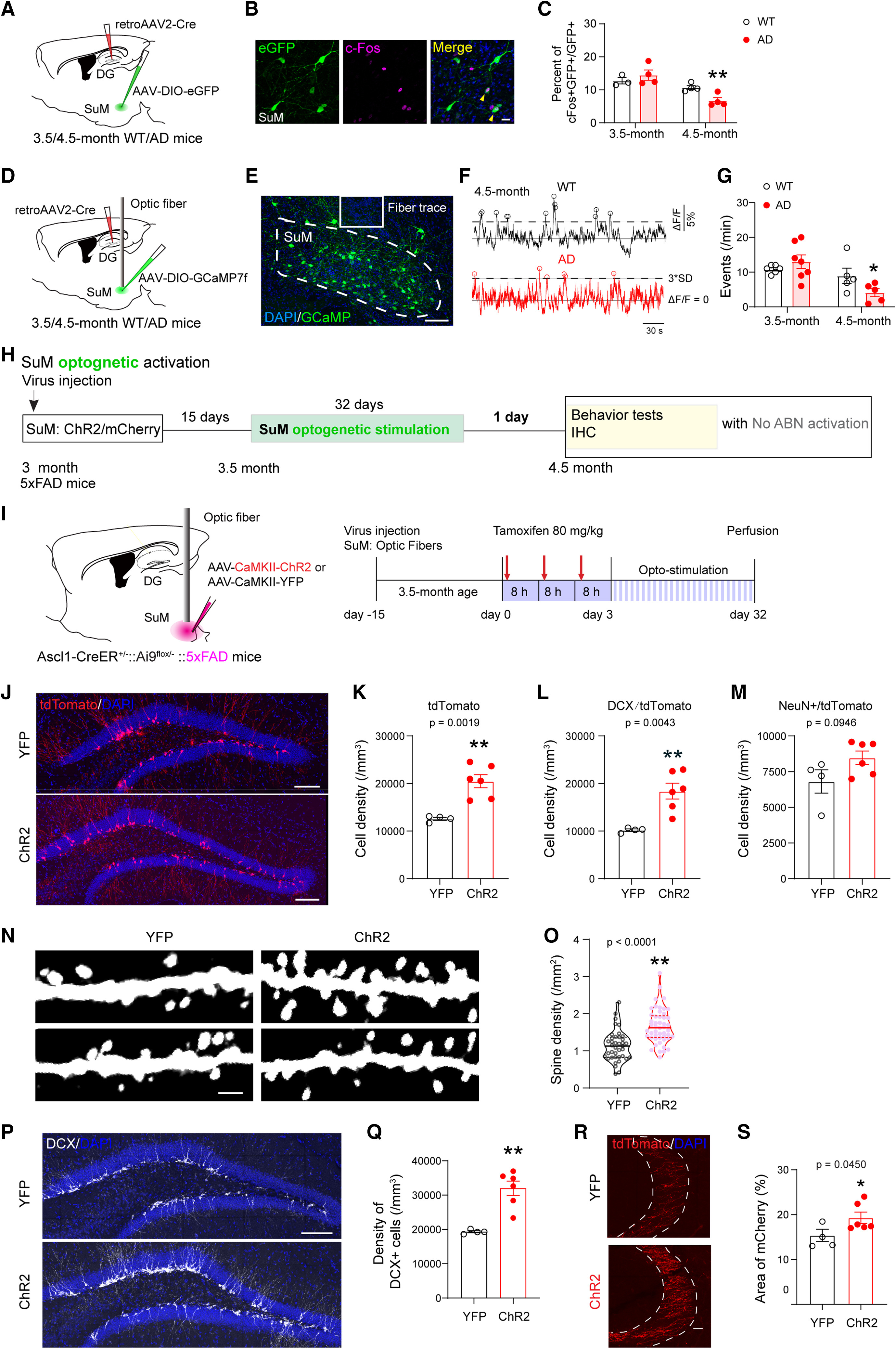
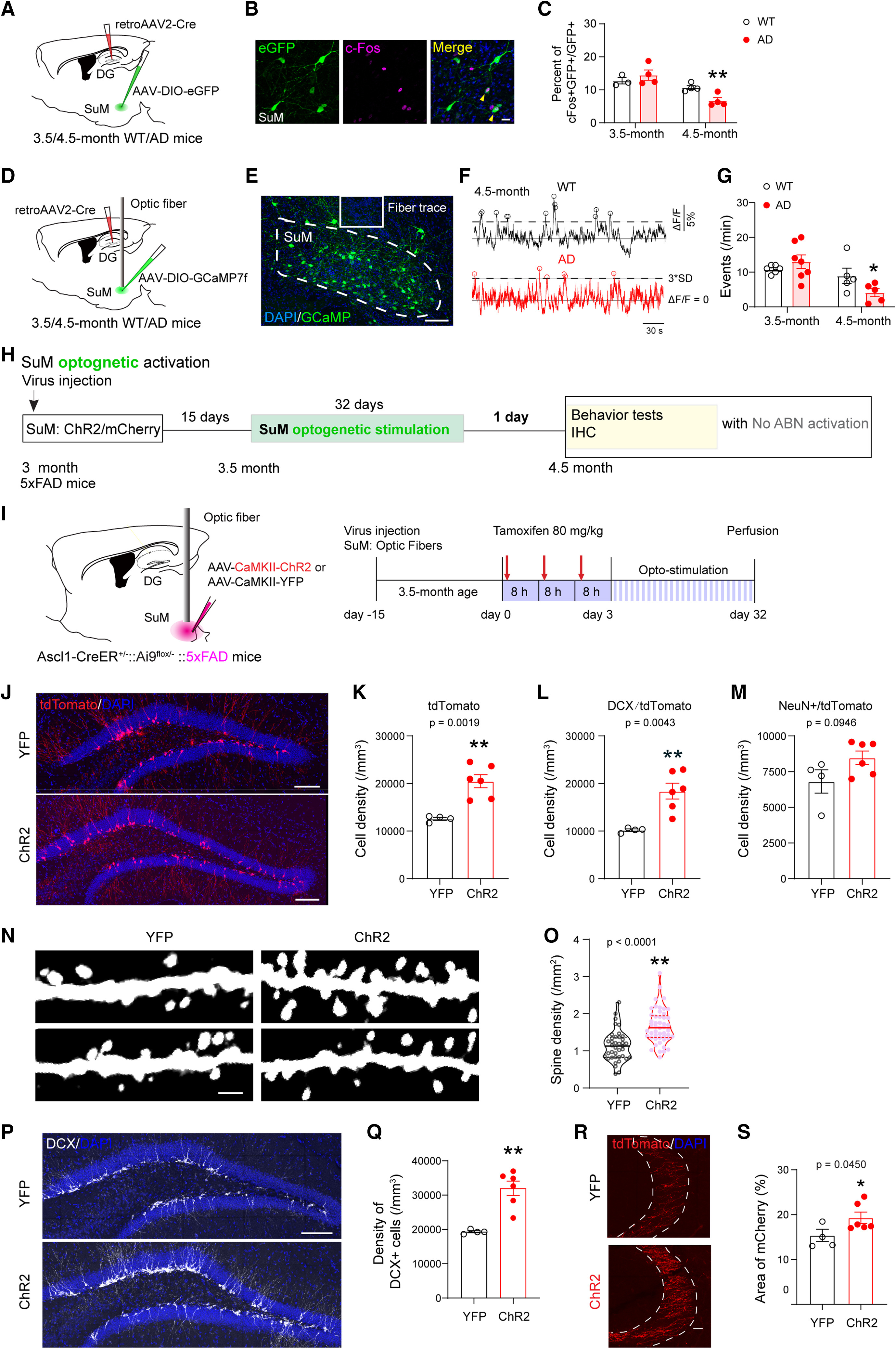
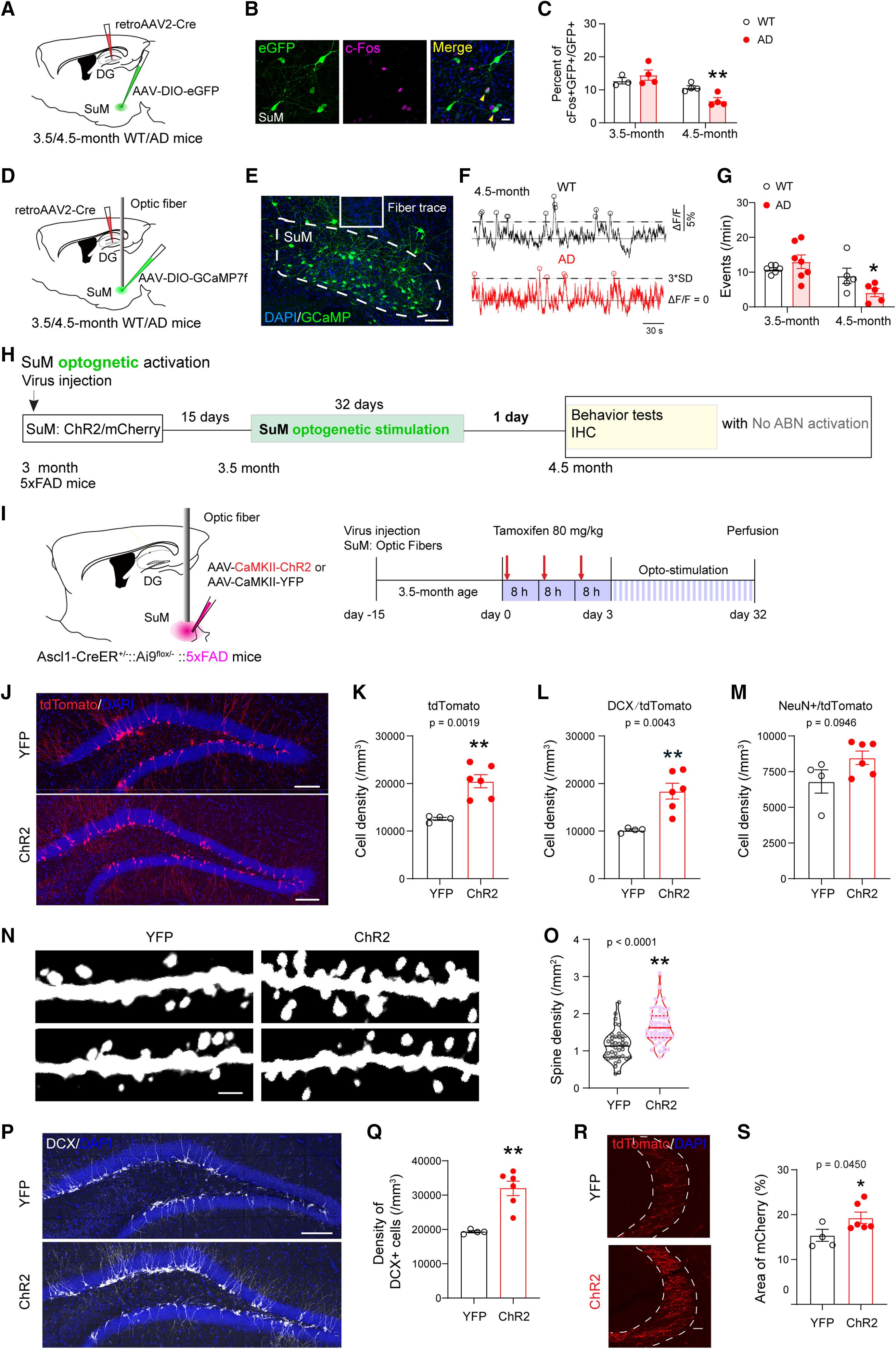
Thus, restoring the ability of newly generated neurons from AHN to incorporate into functional circuitry presents a promising therapeutic approach for AD. A recent study revealed that chemogenetic activation of AHN alone had limited success in reversing hippocampal deficits in 3×Tg-AD and 5×FAD mouse models. However, when paired with optogenetic stimulation of the supramammillary nucleus (SuM, Figure 3a), which projects to the DG and responds actively to pro-neurogenic stimuli, SuM-enhanced adult-born neurons (ABNs, Figure 3b-c) not only improved memory performance but also facilitated the microglial phagocytosis of amyloid plaques [28]. This finding suggests that combining neurogenic stimulation with targeted activation of associated circuitry may enhance integration and functionality of newly formed neurons, potentially offering an effective therapeutic strategy for AD.
| |
|
|
Figure 3: Optogenetic stimulation of SuM enhances Adult-Born Neurons (ABNs) in AD mice. a) Experimental setup showing viral labeling of SuM with ChR2/mCherry and optogenetic stimulation in AD mice for 32 days to restore ABNs. Behavioral tests followed. b) DCX-expressing cells in the dentate gyrus. c) SuM-stimulated ChR2-AD mice showed larger DCX-expressing cell density compared to control YFP-AD mice [28] | |
5. AHN and AD research limitations and future directions
Extensive research has provided valuable insights into AHN and its role in AD, nonetheless, methodological limitations remain. Animal models, particularly mice and rats, are commonly used to explore the association between AHN and AD, but these animals do not naturally develop the disease [reviewed by 29]. Transgenic animals engineered to express human genes associated with genetically inherited FAD—such as mutations in APP and PSEN1 [10, 15, 20, 25, 28, 30-31]—represent only a small subset of human AD cases. Most people suffer from SAD, which lacks clear genetic causes and develops later in life. Additionally, transgenic models tend to exhibit early-onset AD-like pathology, complicating the translation of findings to the late-onset nature of sporadic AD in humans [reviewed by 29]. To address these issues, novel AD models could incorporate environmental risk factors or non-genetic influences of AD, rather than relying solely on genetic factors [32].
Additionally, most human AHN data comes from immunohistochemical studies on postmortem samples [1-2, 8-9]. While AHN is a dynamic process, these fixed-tissue methods may not capture all stages of neurogenesis. Future research should aim to develop non-invasive techniques to study AHN in living humans or animals. For instance, diffusion tensor imaging (DTI) could map white matter changes, indirectly reflecting AHN alterations [33]. Besides, immunohistochemical results from postmortem tissue may also be confounded by variables such as fixation methods, antibody efficiency, and human bias in image analysis. Thus, to confirm AHN reduction in AD, more robust data at the single-cell level is necessary. Single-cell RNA sequencing (scRNA-seq) allows for detailed evaluations of gene expression and cellular diversity. In a recent study, scRNA-seq was used to successfully identify the maturation stages of adult neural stem cells to mature granule cells in aged primates and humans, suggesting the presence of AHN throughout life [34]. Therefore, scRNA-seq could be used to investigate AHN in AD patients compared to healthy controls, providing reliable and quantitative data.
Lastly, although recovering AHN seems a potential method to treat AD, the precise role of newly generated neurons in the adult brain still needs further studies. In 2014, Akers et al. found that high AHN levels lead to forgetting early fear-conditioned memories, while low AHN retains them, suggesting that AHN may facilitate the exchange of old memories for new ones [35]. This raises the concerns that increasing AHN in AD could enhance memory ability but risk older memory loss, possibly worsening AD symptoms. Thus, further research needs to investigate the relationship between AHN and AD and to determine how specifically AHN restoration might contribute to AD symptoms in humans.
6. Conclusion
Research into AHN and AD has advanced our understanding of how impaired neurogenesis may contribute to AD progression. Therapeutic strategies such as stem cell transplantation, modulation of signaling pathways like Wnt/β-catenin, PI3K/Akt, and CREB/BDNF, and stimulation of associated functional circuitry offer promising avenues for restoring AHN and possibly slowing AD progression. Nonetheless, while animal models and human postmortem studies have provided significant insights into disrupted AHN in AD, the exact function of AHN in the disease remains to be fully understood. Further research is necessary to overcome current methodological limitations and develop reliable, non-invasive tools to study AHN in living humans. Ultimately, uncovering the role of AHN in AD pathogenesis holds promising potential for novel early diagnostic markers and effective therapeutic interventions.
References
[1]. AlzBreijyeh, Z., & Karaman, R. (2020). Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules (Basel, Switzerland), 25(24), 5789. https://doi.org/10.3390/molecules25245789
[2]. Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., & Llorens-Martín, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nature medicine, 25(4), 554–560. https://doi.org/10.1038/s41591-019-0375-9
[3]. Kempermann, G., Song, H., & Gage, F. H. (2015). Neurogenesis in the Adult Hippocampus. Cold Spring Harbor perspectives in biology, 7(9), a018812. https://doi.org/10.1101/cshperspect.a018812
[4]. Ramon y Cajal, S. (1913) Degeneration and regeneration of the nervous system. Oxford Univ Press, London, 1913.
[5]. Altman, J., & Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of comparative neurology, 124(3), 319–335. https://doi.org/10.1002/cne.901240303
[6]. Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature medicine, 4(11), 1313–1317. https://doi.org/10.1038/3305
[7]. Sorrells, S. F., Paredes, M. F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K. W., James, D., Mayer, S., Chang, J., Auguste, K. I., Chang, E. F., Gutierrez, A. J., Kriegstein, A. R., Mathern, G. W., Oldham, M. C., Huang, E. J., Garcia-Verdugo, J. M., Yang, Z., & Alvarez-Buylla, A. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 555(7696), 377–381. https://doi.org/10.1038/nature25975
[8]. Cao, Y., Liu, P., Bian, H., Jin, S., Liu, J., Yu, N., Cui, H., Sun, F., Qian, X., Qiu, W., & Ma, C. (2024). Reduced neurogenesis in human hippocampus with Alzheimer's disease. Brain pathology (Zurich, Switzerland), 34(3), e13225. https://doi.org/10.1111/bpa.13225
[9]. Tobin, M. K., Musaraca, K., Disouky, A., Shetti, A., Bheri, A., Honer, W. G., Kim, N., Dawe, R. J., Bennett, D. A., Arfanakis, K., & Lazarov, O. (2019). Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer's Disease Patients. Cell stem cell, 24(6), 974–982.e3. https://doi.org/10.1016/j.stem.2019.05.003
[10]. Unger, M. S., Marschallinger, J., Kaindl, J., Höfling, C., Rossner, S., Heneka, M. T., Van der Linden, A., & Aigner, L. (2016). Early Changes in Hippocampal Neurogenesis in Transgenic Mouse Models for Alzheimer's Disease. Molecular neurobiology, 53(8), 5796–5806. https://doi.org/10.1007/s12035-016-0018-9
[11]. Rudnitskaya, E. A., Kozlova, T. A., Burnyasheva, A. O., Kolosova, N. G., & Stefanova, N. A. (2019). Alterations of hippocampal neurogenesis during development of Alzheimer's disease-like pathology in OXYS rats. Experimental gerontology, 115, 32–45. https://doi.org/10.1016/j.exger.2018.11.008
[12]. Lee, I. S., Jung, K., Kim, I. S., Lee, H., Kim, M., Yun, S., Hwang, K., Shin, J. E., & Park, K. I. (2015). Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Molecular neurodegeneration, 10, 38. https://doi.org/10.1186/s13024-015-0035-6
[13]. Cheng, Q., Ma, X., Liu, J., Feng, X., Liu, Y., Wang, Y., Ni, W., & Song, M. (2023). Pharmacological Inhibition of the Asparaginyl Endopeptidase (AEP) in an Alzheimer's Disease Model Improves the Survival and Efficacy of Transplanted Neural Stem Cells. International journal of molecular sciences, 24(9), 7739. https://doi.org/10.3390/ijms24097739
[14]. Liu, J., Xiao, Q., Xiao, J., Niu, C., Li, Y., Zhang, X., Zhou, Z., Shu, G., & Yin, G. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal transduction and targeted therapy, 7(1), 3. https://doi.org/10.1038/s41392-021-00762-6
[15]. He, P., & Shen, Y. (2009). Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer's disease. The Journal of neuroscience: the official journal of the Society for Neuroscience, 29(20), 6545–6557. https://doi.org/10.1523/JNEUROSCI.0421-09.2009
[16]. Liu, C. C., Tsai, C. W., Deak, F., Rogers, J., Penuliar, M., Sung, Y. M., Maher, J. N., Fu, Y., Li, X., Xu, H., Estus, S., Hoe, H. S., Fryer, J. D., Kanekiyo, T., & Bu, G. (2014). Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer's disease. Neuron, 84(1), 63–77. https://doi.org/10.1016/j.neuron.2014.08.048
[17]. Wang, Q., Huang, X., Su, Y., Yin, G., Wang, S., Yu, B., Li, H., Qi, J., Chen, H., Zeng, W., Zhang, K., Verkhratsky, A., Niu, J., & Yi, C. (2022). Activation of Wnt/β-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer's disease. Brain: a journal of neurology, 145(12), 4474–4488. https://doi.org/10.1093/brain/awac236
[18]. Jones, M. E., Büchler, J., Dufor, T., Palomer, E., Teo, S., Martin-Flores, N., Boroviak, K., Metzakopian, E., Gibb, A., & Salinas, P. C. (2023). A genetic variant of the Wnt receptor LRP6 accelerates synapse degeneration during aging and in Alzheimer's disease. Science advances, 9(2), eabo7421. https://doi.org/10.1126/sciadv.abo7421
[19]. Santos, S. S., Moreira, J. B., Costa, M., Rodrigues, R. S., Sebastião, A. M., Xapelli, S., & Solá, S. (2021). The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells, 11(1), 90. https://doi.org/10.3390/cells11010090
[20]. Dai, N., Su, X., Li, A., Li, J., Jiang, D., & Wang, Y. (2024). DVL/GSK3/ISL1 pathway signaling: unraveling the mechanism of SIRT3 in neurogenesis and AD therapy. Stem cell research & therapy, 15(1), 299. https://doi.org/10.1186/s13287-024-03925-8
[21]. Razani, E., Pourbagheri-Sigaroodi, A., Safaroghli-Azar, A., Zoghi, A., Shanaki-Bavarsad, M., & Bashash, D. (2021). The PI3K/Akt signaling axis in Alzheimer's disease: a valuable target to stimulate or suppress?. Cell stress & chaperones, 26(6), 871–887. https://doi.org/10.1007/s12192-021-01231-3
[22]. Park, H. J., Kwon, H., Lee, J. H., Cho, E., Lee, Y. C., Moon, M., Jun, M., Kim, D. H., & Jung, J. W. (2020). β-Amyrin Ameliorates Alzheimer's Disease-Like Aberrant Synaptic Plasticity in the Mouse Hippocampus. Biomolecules & therapeutics, 28(1), 74–82. https://doi.org/10.4062/biomolther.2019.024
[23]. Miranda, M., Morici, J. F., Zanoni, M. B., & Bekinschtein, P. (2019). Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Frontiers in cellular neuroscience, 13, 363. https://doi.org/10.3389/fncel.2019.00363
[24]. Peng, S., Wuu, J., Mufson, E. J., & Fahnestock, M. (2005). Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. Journal of neurochemistry, 93(6), 1412–1421. https://doi.org/10.1111/j.1471-4159.2005.03135.x
[25]. She, L., Tang, H., Zeng, Y., Li, L., Xiong, L., Sun, J., Chen, F., Ren, J., Zhang, J., Wang, W., Zhao, X., & Liang, G. (2024). Ginsenoside RK3 promotes neurogenesis in Alzheimer's disease through activation of the CREB/BDNF pathway. Journal of ethnopharmacology, 321, 117462. https://doi.org/10.1016/j.jep.2023.117462
[26]. Lou, S., Gong, D., Yang, M., Qiu, Q., Luo, J., & Chen, T. (2024). Curcumin Improves Neurogenesis in Alzheimer's Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. International journal of molecular sciences, 25(10), 5123. https://doi.org/10.3390/ijms25105123
[27]. van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D., & Gage, F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature, 415(6875), 1030–1034. https://doi.org/10.1038/4151030a
[28]. Li, Y. D., Luo, Y. J., Xie, L., Tart, D. S., Sheehy, R. N., Zhang, L., Coleman, L. G., Jr, Chen, X., & Song, J. (2023). Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer's disease. Cell stem cell, 30(4), 415–432.e6. https://doi.org/10.1016/j.stem.2023.02.006
[29]. Granzotto, A., Vissel, B., Sensi S. L. (2024). Lost in translation: Inconvenient truths on the utility of mouse models in Alzheimer’s disease research. eLife, 13:e90633. https://doi.org/10.7554/eLife.90633
[30]. Zeng, Q., Zheng, M., Zhang, T., & He, G. (2016). Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer's disease. Neuroscience, 314, 64–74. https://doi.org/10.1016/j.neuroscience.2015.11.054
[31]. Zhang, C., McNeil, E., Dressler, L., & Siman, R. (2007). Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Experimental neurology, 204(1), 77–87. https://doi.org/10.1016/j.expneurol.2006.09.018
[32]. Ganesan, K., Rentsch, P., Langdon, A., Milham, L. T., & Vissel, B. (2024). Modeling sporadic Alzheimer's disease in mice by combining Apolipoprotein E4 risk gene with environmental risk factors. Frontiers in aging neuroscience, 16, 1357405. https://doi.org/10.3389/fnagi.2024.1357405
[33]. Islam, M. R., Luo, R., Valaris, S., Haley, E. B., Takase, H., Chen, Y. I., Dickerson, B. C., Schon, K., Arai, K., Nguyen, C. T., & Wrann, C. D. (2020). Diffusion tensor-MRI detects exercise-induced neuroplasticity in the hippocampal microstructure in mice. Brain plasticity (Amsterdam, Netherlands), 5(2), 147–159. https://doi.org/10.3233/BPL-190090
[34]. Wang, W., Wang, M., Yang, M., Zeng, B., Qiu, W., Ma, Q., Jing, X., Zhang, Q., Wang, B., Yin, C., Zhang, J., Ge, Y., Lu, Y., Ji, W., Wu, Q., Ma, C., & Wang, X. (2022). Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell research, 32(8), 729–743. https://doi.org/10.1038/s41422-022-00678-y
[35]. Akers, K. G., Martinez-Canabal, A., Restivo, L., Yiu, A. P., De Cristofaro, A., Hsiang, H. L., Wheeler, A. L., Guskjolen, A., Niibori, Y., Shoji, H., Ohira, K., Richards, B. A., Miyakawa, T., Josselyn, S. A., & Frankland, P. W. (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science (New York, N.Y.), 344(6184), 598–602. https://doi.org/10.1126/science.1248903
Cite this article
Yang,J. (2025). The Role of Adult Hippocampal Neurogenesis in Alzheimer's Disease: Mechanisms, Therapeutic Potential, and Future Directions. Theoretical and Natural Science,115,26-33.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. AlzBreijyeh, Z., & Karaman, R. (2020). Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules (Basel, Switzerland), 25(24), 5789. https://doi.org/10.3390/molecules25245789
[2]. Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., & Llorens-Martín, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nature medicine, 25(4), 554–560. https://doi.org/10.1038/s41591-019-0375-9
[3]. Kempermann, G., Song, H., & Gage, F. H. (2015). Neurogenesis in the Adult Hippocampus. Cold Spring Harbor perspectives in biology, 7(9), a018812. https://doi.org/10.1101/cshperspect.a018812
[4]. Ramon y Cajal, S. (1913) Degeneration and regeneration of the nervous system. Oxford Univ Press, London, 1913.
[5]. Altman, J., & Das, G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of comparative neurology, 124(3), 319–335. https://doi.org/10.1002/cne.901240303
[6]. Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature medicine, 4(11), 1313–1317. https://doi.org/10.1038/3305
[7]. Sorrells, S. F., Paredes, M. F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K. W., James, D., Mayer, S., Chang, J., Auguste, K. I., Chang, E. F., Gutierrez, A. J., Kriegstein, A. R., Mathern, G. W., Oldham, M. C., Huang, E. J., Garcia-Verdugo, J. M., Yang, Z., & Alvarez-Buylla, A. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 555(7696), 377–381. https://doi.org/10.1038/nature25975
[8]. Cao, Y., Liu, P., Bian, H., Jin, S., Liu, J., Yu, N., Cui, H., Sun, F., Qian, X., Qiu, W., & Ma, C. (2024). Reduced neurogenesis in human hippocampus with Alzheimer's disease. Brain pathology (Zurich, Switzerland), 34(3), e13225. https://doi.org/10.1111/bpa.13225
[9]. Tobin, M. K., Musaraca, K., Disouky, A., Shetti, A., Bheri, A., Honer, W. G., Kim, N., Dawe, R. J., Bennett, D. A., Arfanakis, K., & Lazarov, O. (2019). Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer's Disease Patients. Cell stem cell, 24(6), 974–982.e3. https://doi.org/10.1016/j.stem.2019.05.003
[10]. Unger, M. S., Marschallinger, J., Kaindl, J., Höfling, C., Rossner, S., Heneka, M. T., Van der Linden, A., & Aigner, L. (2016). Early Changes in Hippocampal Neurogenesis in Transgenic Mouse Models for Alzheimer's Disease. Molecular neurobiology, 53(8), 5796–5806. https://doi.org/10.1007/s12035-016-0018-9
[11]. Rudnitskaya, E. A., Kozlova, T. A., Burnyasheva, A. O., Kolosova, N. G., & Stefanova, N. A. (2019). Alterations of hippocampal neurogenesis during development of Alzheimer's disease-like pathology in OXYS rats. Experimental gerontology, 115, 32–45. https://doi.org/10.1016/j.exger.2018.11.008
[12]. Lee, I. S., Jung, K., Kim, I. S., Lee, H., Kim, M., Yun, S., Hwang, K., Shin, J. E., & Park, K. I. (2015). Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Molecular neurodegeneration, 10, 38. https://doi.org/10.1186/s13024-015-0035-6
[13]. Cheng, Q., Ma, X., Liu, J., Feng, X., Liu, Y., Wang, Y., Ni, W., & Song, M. (2023). Pharmacological Inhibition of the Asparaginyl Endopeptidase (AEP) in an Alzheimer's Disease Model Improves the Survival and Efficacy of Transplanted Neural Stem Cells. International journal of molecular sciences, 24(9), 7739. https://doi.org/10.3390/ijms24097739
[14]. Liu, J., Xiao, Q., Xiao, J., Niu, C., Li, Y., Zhang, X., Zhou, Z., Shu, G., & Yin, G. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal transduction and targeted therapy, 7(1), 3. https://doi.org/10.1038/s41392-021-00762-6
[15]. He, P., & Shen, Y. (2009). Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer's disease. The Journal of neuroscience: the official journal of the Society for Neuroscience, 29(20), 6545–6557. https://doi.org/10.1523/JNEUROSCI.0421-09.2009
[16]. Liu, C. C., Tsai, C. W., Deak, F., Rogers, J., Penuliar, M., Sung, Y. M., Maher, J. N., Fu, Y., Li, X., Xu, H., Estus, S., Hoe, H. S., Fryer, J. D., Kanekiyo, T., & Bu, G. (2014). Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer's disease. Neuron, 84(1), 63–77. https://doi.org/10.1016/j.neuron.2014.08.048
[17]. Wang, Q., Huang, X., Su, Y., Yin, G., Wang, S., Yu, B., Li, H., Qi, J., Chen, H., Zeng, W., Zhang, K., Verkhratsky, A., Niu, J., & Yi, C. (2022). Activation of Wnt/β-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer's disease. Brain: a journal of neurology, 145(12), 4474–4488. https://doi.org/10.1093/brain/awac236
[18]. Jones, M. E., Büchler, J., Dufor, T., Palomer, E., Teo, S., Martin-Flores, N., Boroviak, K., Metzakopian, E., Gibb, A., & Salinas, P. C. (2023). A genetic variant of the Wnt receptor LRP6 accelerates synapse degeneration during aging and in Alzheimer's disease. Science advances, 9(2), eabo7421. https://doi.org/10.1126/sciadv.abo7421
[19]. Santos, S. S., Moreira, J. B., Costa, M., Rodrigues, R. S., Sebastião, A. M., Xapelli, S., & Solá, S. (2021). The Mitochondrial Antioxidant Sirtuin3 Cooperates with Lipid Metabolism to Safeguard Neurogenesis in Aging and Depression. Cells, 11(1), 90. https://doi.org/10.3390/cells11010090
[20]. Dai, N., Su, X., Li, A., Li, J., Jiang, D., & Wang, Y. (2024). DVL/GSK3/ISL1 pathway signaling: unraveling the mechanism of SIRT3 in neurogenesis and AD therapy. Stem cell research & therapy, 15(1), 299. https://doi.org/10.1186/s13287-024-03925-8
[21]. Razani, E., Pourbagheri-Sigaroodi, A., Safaroghli-Azar, A., Zoghi, A., Shanaki-Bavarsad, M., & Bashash, D. (2021). The PI3K/Akt signaling axis in Alzheimer's disease: a valuable target to stimulate or suppress?. Cell stress & chaperones, 26(6), 871–887. https://doi.org/10.1007/s12192-021-01231-3
[22]. Park, H. J., Kwon, H., Lee, J. H., Cho, E., Lee, Y. C., Moon, M., Jun, M., Kim, D. H., & Jung, J. W. (2020). β-Amyrin Ameliorates Alzheimer's Disease-Like Aberrant Synaptic Plasticity in the Mouse Hippocampus. Biomolecules & therapeutics, 28(1), 74–82. https://doi.org/10.4062/biomolther.2019.024
[23]. Miranda, M., Morici, J. F., Zanoni, M. B., & Bekinschtein, P. (2019). Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Frontiers in cellular neuroscience, 13, 363. https://doi.org/10.3389/fncel.2019.00363
[24]. Peng, S., Wuu, J., Mufson, E. J., & Fahnestock, M. (2005). Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. Journal of neurochemistry, 93(6), 1412–1421. https://doi.org/10.1111/j.1471-4159.2005.03135.x
[25]. She, L., Tang, H., Zeng, Y., Li, L., Xiong, L., Sun, J., Chen, F., Ren, J., Zhang, J., Wang, W., Zhao, X., & Liang, G. (2024). Ginsenoside RK3 promotes neurogenesis in Alzheimer's disease through activation of the CREB/BDNF pathway. Journal of ethnopharmacology, 321, 117462. https://doi.org/10.1016/j.jep.2023.117462
[26]. Lou, S., Gong, D., Yang, M., Qiu, Q., Luo, J., & Chen, T. (2024). Curcumin Improves Neurogenesis in Alzheimer's Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. International journal of molecular sciences, 25(10), 5123. https://doi.org/10.3390/ijms25105123
[27]. van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D., & Gage, F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature, 415(6875), 1030–1034. https://doi.org/10.1038/4151030a
[28]. Li, Y. D., Luo, Y. J., Xie, L., Tart, D. S., Sheehy, R. N., Zhang, L., Coleman, L. G., Jr, Chen, X., & Song, J. (2023). Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer's disease. Cell stem cell, 30(4), 415–432.e6. https://doi.org/10.1016/j.stem.2023.02.006
[29]. Granzotto, A., Vissel, B., Sensi S. L. (2024). Lost in translation: Inconvenient truths on the utility of mouse models in Alzheimer’s disease research. eLife, 13:e90633. https://doi.org/10.7554/eLife.90633
[30]. Zeng, Q., Zheng, M., Zhang, T., & He, G. (2016). Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer's disease. Neuroscience, 314, 64–74. https://doi.org/10.1016/j.neuroscience.2015.11.054
[31]. Zhang, C., McNeil, E., Dressler, L., & Siman, R. (2007). Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Experimental neurology, 204(1), 77–87. https://doi.org/10.1016/j.expneurol.2006.09.018
[32]. Ganesan, K., Rentsch, P., Langdon, A., Milham, L. T., & Vissel, B. (2024). Modeling sporadic Alzheimer's disease in mice by combining Apolipoprotein E4 risk gene with environmental risk factors. Frontiers in aging neuroscience, 16, 1357405. https://doi.org/10.3389/fnagi.2024.1357405
[33]. Islam, M. R., Luo, R., Valaris, S., Haley, E. B., Takase, H., Chen, Y. I., Dickerson, B. C., Schon, K., Arai, K., Nguyen, C. T., & Wrann, C. D. (2020). Diffusion tensor-MRI detects exercise-induced neuroplasticity in the hippocampal microstructure in mice. Brain plasticity (Amsterdam, Netherlands), 5(2), 147–159. https://doi.org/10.3233/BPL-190090
[34]. Wang, W., Wang, M., Yang, M., Zeng, B., Qiu, W., Ma, Q., Jing, X., Zhang, Q., Wang, B., Yin, C., Zhang, J., Ge, Y., Lu, Y., Ji, W., Wu, Q., Ma, C., & Wang, X. (2022). Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell research, 32(8), 729–743. https://doi.org/10.1038/s41422-022-00678-y
[35]. Akers, K. G., Martinez-Canabal, A., Restivo, L., Yiu, A. P., De Cristofaro, A., Hsiang, H. L., Wheeler, A. L., Guskjolen, A., Niibori, Y., Shoji, H., Ohira, K., Richards, B. A., Miyakawa, T., Josselyn, S. A., & Frankland, P. W. (2014). Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science (New York, N.Y.), 344(6184), 598–602. https://doi.org/10.1126/science.1248903