1. Introduction
Cancers have consistently represented a significant challenge for medical research, representing one of the most prevalent and life-threatening diseases affecting human health. Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer mortality and the third most common cause of cancer-related death. While current clinical treatment and drug therapy can reduce morbidity and mortality, chemotherapy and anti-tumour drugs have strong side effects and can significantly impair the patient's immune-regulating ability. The majority of cases of hepatocellular carcinoma are considered incurable due to the extensive dysfunction of the liver resulting from combined cirrhosis, the rarity of early diagnosis, and the lack of appropriate chemotherapy. However, a number of studies demonstrated that the risk of HCC might be reduced by Andrograhpolide, a major ingredient of Traditional Chinese Medicine, that can induce senescence in human hepatocellular carcinoma cells HepG2 via p53/p21.
Apoptosis represents a physiological process that results in the loss of cells and serves to maintain equilibrium between cell proliferation and death. Cancer cells are distinguished in the regulation of cell proliferation. The growth of tumours is contingent upon the rate of cell proliferation and apoptosis. Andrographolide has been demonstrated to exert anti-tumour effects by inducing cell cycle via p53/p21 in G1-S phase arrest, which ultimately results in apoptosis. In the cell cycle, the regulatory function of P53 is mainly reflected in the monitoring of G1 and S-phase correction points, which is closely related to transcriptional activation.The protein encoded by P53 downstream gene P21 is a Cyclin-dependent protein kinase inhibitor.P21 can bind to a series of Cyclin-cdk complexes and inhibit the activity of the corresponding protein kinase, resulting in Cyclin-cdk not being able to phosphorylate Rb, and the non-phosphorylated Rb keeps binding to E2F, which prevents E2F, a transcriptional regulator, from being activated, and causes the G1 block [1].
Andrographolide is a dicyclic diterpene lactone extracted from Andrographis paniculata with significant anti-tumour activity associated with the modulation of various signalling pathways.In general, andrographolide is relatively safe although it may have some toxicity [2].
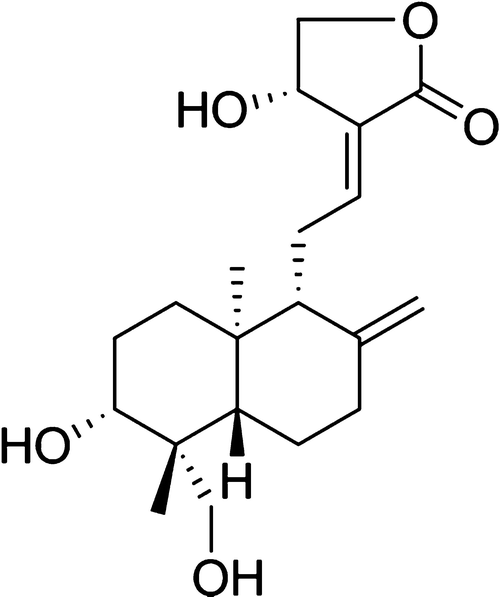
Figure 1: The structure of Andrographolide
(2-(Decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenenaphthyl)ethylidene)dihydro-4-hydroxyfuran-2(3H)-one [3]
The aim of the present study was to examine increasing concentrations and treatment durations with Andrographolide is able to induce senescence in human hepatocellular carcinoma HEPG2 cells through an increase in p21 and p53 resulting in decreased HEPg2 viability.
2. Material and methods
2.1. Reagent
Andrographolide was dissolved in dimethyl sulfoxide and kept at − 20C before use. The purity of andrographolide is 98%.
2.2. Cell culture
The cells were added into a 15ml centrifuge tube containing preheated medium and centrifuged at 1000rpm for 5 mins. The supernatant is aspirated to obtain cell precipitation, and the cells are gently re-suspended with 2ml complete medium (basic medium +FBS+double antibody) and added to a 6-well microplate for culture in a moderate incubator saturated with 5%CO2 at 37℃.Cells were subjected to 0.01, 0.1, and 1 g mL-1 of AD supplied at the beginning (0h) and after 48 hours of the 72-hour culture to evaluate the influence of AD.
2.3. Measure senesscence by by b-gal kit
For cells cultured in 6-well plates, the cell culture medium was sucked, washed with PBS once, and 1mL Fixative Solution was added, and fixed at room temperature for 15min. The cells were washed with PBS 3 times for 3min each time. PBS was sucked and 1mL Staining Solution was added to each well (50µL X-gal Solution, 10µL Solution A, 10µL Solution B, 930µL Staining buffer was extracted and mixed). Incubation at 37ºC overnight, and finally observation and counting under ordinary light microscope.
2.4. Measure abundance of p21 and p53 by western blot
1. Extraction of total cellular protein
Melt the RIPA lysate and mix well. Take appropriate amount of lysate and add PMSF within minutes before use to make the final concentration of PMSF 1 mM. Add lysate into 6-well plate with adherent cells to make full contact between lysate and cells.
2. Determination of protein content
Protein content was determined using the BCA kit.
3. SDS-PAGE electrophoresis
4. Transferring membrane
Transfer the gel to PVDF membrane for electrotransferring.
5. Membrane closure and antibody incubation
Transfer to a flat dish containing the sealing solution, shake at room temperature on a decolourising shaker for 1 hour, then wash with TBS 3 times for 10 minutes each time. Add the prepared primary antibody, to the corresponding membrane, and incubate at 4℃ overnight.
6. Chemiluminescence
Luminescent solution (liquid A: liquid B) 1:1 configuration (pay attention to avoid light), pipette the appropriate amount of luminescent solution to cover the PVDF membrane, ECL luminescence instrument exposure and image acquisition.
2.5. Measure cell apoptosis analysis by tunnel assay
Fluorescein (FITC) -labeled dUTP (fluorescein (dUTP)) can be added with the catalysis of terminal deoxynucleotide transferase when the genomic DNA is broken and can be examined by fluorescence microscopy or flow cytometry. All treatment groups were compared with a control group that received no treatment.
3. Results
3.1. AD increases senescence of HepG2
Possible result 1: AD can induce senescence of HepG2 at low concentration
In the culture of HepG2 cells, the addition of AD may induce the acceleration of cell decay and lead to senescence.
Possible outcome 2: AD does not induce senescence in HepG2 at tested concentrations
HepG2 did not respond to AD at the detection level. It does not induce accelerated cell decay, eliminating the possibility that they may be helping to treat hepatocellular carcinoma.
3.2. AD increase p21 by western blot
Possible result 3: AD increased p21 content in HepG2
p21 inhibits the corresponding protease activity and causes G1 phase arrest
Possible result 4: p21 expression did not change significantly
AD does not interact directly with p21
3.3. AD increases p53 by western blot
Possible result 5: AD increased p53 content in HepG2
P53 can complete the regulation of apoptosis and participate in the process of DNA repair. Its DNA binding domain itself has the activity of endonuclease, which can cut out mismatched nucleotides, and the binding well regulates the activity of endonucleotide repair factors XPB and XPD, thus affecting its DNA recombination and repair functions.
Possible result 6: p53 expression did not change significantly
AD does not interact directly with p53
3.4. AD decreases HepG2 viability
Possible outcome 7: Decreased HepG2 viability accelerated cell death
Possible Outcome 8: No significant change in the HepG2 viability
Table 1: AD decreases HepG2 viability
Combination Result # (CR#) | |||||
AD increases senescence by b-gal assay | AD increase p21 by western blot | AD increases p53 by western blot | AD decreases HepG2 viability by MTT | Support of hypothesis | |
1 | + | + | + | + | Full |
2 | + | + | + | - | Partial |
3 | + | + | - | + | Partial |
4 | + | - | + | + | Partial |
5 | - | + | + | + | Partial |
6 | + | + | - | - | Partial |
7 | + | - | - | + | Partial |
8 | - | - | + | + | Partial |
9 | + | - | + | - | Partial |
10 | - | + | - | + | Partial |
11 | - | + | + | - | Partial |
12 | + | - | - | - | Partial |
13 | - | + | - | - | Partial |
14 | - | - | + | - | Partial |
15 | - | - | - | + | Partial |
16 | - | - | - | - | Fully Contradicts |
Table legend: "+" indicates AD express efficacy , "-" indicates AD not express efficacy
4. Discussion
Previous studies have demonstrated that AD contributes to human lung cell carcinoma, and AD can induce lung cell cancer cell senescence through p21/p53 and Skp2/p27. They explain the mechanism by which AD induces senescence in lung cells and cancer cells.4 p52/p21 plays a crucial role in regulating cell cycle arrest and induction [5].
CR1 is fully consistent with the hypothesis that AD will increase aging of HepG2, increase p52/p21 content and decrease HepG2 viability. p53/p21 can block and induce cell cycle in G1-S phase and eventually lead to apoptosis, thus playing an anti-tumor role.
CR2 partially conforms to the hypothesis that AD does not reduce HepG2 viability, resulting in HepG2 not accelerating death [6], reducing the possibility of anti-tumor. If the concentration of AD can be increased, HepG2 may decrease the activity more.
CR3,4,7 are in line with our hypothesis that AD cannot increase the content of p53/p21, blocking both p53 and p21, and cells lacking p53 cannot stop [7].CR5 exemplifies that AD, although leading to increased levels of p21 and p53, is not able to reduce HepG2 senescence.
CR6 exemplifies that AD is able to increase HepG2 senescence but not reduce viability. p21 activation alone in the absence of p53 may promote cancer development.
CR8 is partially consistent with hypothesis that the p53 number is increased but the p21 is not affected, suggesting that p53 is not successful in inducing the transcription of p21. This also leads to the inability of AD to reduce HepG2 senescence.
CR9 indicated an increase in p53 and blocked the cell cycle in G1-G2/M phase, leading to HepG2 senescence. However, AD could not affect the viability of HepG2.
CR10 is partially in line with my hypothesis that AD has a role in decreasing HepG2 viability,but does not induce HepG2 senescence.
CR11 partially fits hypothesis that both p21 and p53 increase in response to AD. However, p21 and p53 were not successful in inducing HepG2 senescence.
CR12 demonstrated increased HepG2 senescence in the absence of increased p21 and p53. There have possibility of other factors contributing to HepG2 senescence.
CR13 is not very consistent with the hypothesis that AD does not induce HepG2 senescence nor does it decrease HepG2 viability with only an increased amount of p21. p21 activation alone can lead to faster cancer progression.
CR14 is less consistent with the hypothesis that only the amount of p53 is increase and that without enough p21, p53 cannot bind the promoter of the p21 gene and induce transcription of the p21 gene, thus indirectly organising the cell cycle and induce HepG2 senescence.
CR15 is partially in line with the hypothesis that the viability of HepG2 is reduce and is able to have a partial effect towards resisting cancer cell replication.
CR16 is completely inconsistent with the hypothesis that AD cannot increase the content of p53/p21, AD cannot increase cell senescence and reduce the viability of HepG2. My opinion on this result is that the concentration of AD needs to be increased. According to previous studies, AD induces G0/G1 phase to block the expression of p53, p21 and p16 in human colorectal cancer cell lines by up-regulating the G0/G1 phase [8].AD induced G0/G1 block of A549 cells and G2/M block of NCI-H1975 cells. These results suggest that AD induces cell cycle arrest in a cell-type-specific manner. Therefore, the content of p53/p21 will increase [4].
5. Conclusions
In conclusions, this study explores the effects of Andrograhpolide on human hepatocellular cancer cells HepG2. Studies have shown that Andrograhpolide can induce HepG2 senescence and delay tumor formation through p21/p53, thus reducing the risk of HCC.
References
[1]. Shats I, Milyavsky M, Tang X, et al. p53-dependent down-regulation of telomerase is mediated by p21waf1[J]. Journal of Biological Chemistry, 2004, 279(49): 50976-50985.
[2]. Lu J, Ma Y, Wu J, et al. A review for the neuroprotective effects of andrographolide in the central nervous system[J]. Biomedicine & Pharmacotherapy, 2019, 117: 109078.
[3]. Yan, Y., Fang, LH., Du, GH. (2018). Andrographolide. In: Natural Small Molecule Drugs from Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-8022-7_60
[4]. Zhang J, Li C, Zhang L, et al. Andrographolide, a diterpene lactone from the Traditional Chinese Medicine Andrographis paniculate, induces senescence in human lung adenocarcinoma via p53/p21 and Skp2/p27[J]. Phytomedicine, 2022, 98: 153933.
[5]. Collado M, Blasco M A, Serrano M. Cellular senescence in cancer and aging[J]. Cell, 2007, 130(2): 223-233.
[6]. Mark A. Tirmenstein, Catherine X. Hu, Tracy L. Gales, Beverly E. Maleeff, Padma K. Narayanan, Edit Kurali, Timothy K. Hart, Heath C. Thomas, Lester W. Schwartz, Effects of Troglitazone on HepG2 Viability and Mitochondrial Function, Toxicological Sciences, Volume 69, Issue 1, September 2002, Pages 131–138, https://doi.org/10.1093/toxsci/69.1.131
[7]. Mikule K, Delaval B, Kaldis P, et al. Loss of centrosome integrity induces p38—p53—p21-dependent G1—S arrest[J]. Nature cell biology, 2007, 9(2): 160-170.
[8]. Shi, M.D., Lin, H.H., Lee, Y.C., Chao, J.K., Lin, R.A., Chen, J.H., 2008. Inhibition of cellcycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem. Biol. Interact. 174, 201–210.
Cite this article
Zhou,X. (2025). The Effect of Andrographolide on Induce Senescence in Human Hepatocellular Carcinoma HepG2 Cells. Theoretical and Natural Science,115,66-71.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Shats I, Milyavsky M, Tang X, et al. p53-dependent down-regulation of telomerase is mediated by p21waf1[J]. Journal of Biological Chemistry, 2004, 279(49): 50976-50985.
[2]. Lu J, Ma Y, Wu J, et al. A review for the neuroprotective effects of andrographolide in the central nervous system[J]. Biomedicine & Pharmacotherapy, 2019, 117: 109078.
[3]. Yan, Y., Fang, LH., Du, GH. (2018). Andrographolide. In: Natural Small Molecule Drugs from Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-8022-7_60
[4]. Zhang J, Li C, Zhang L, et al. Andrographolide, a diterpene lactone from the Traditional Chinese Medicine Andrographis paniculate, induces senescence in human lung adenocarcinoma via p53/p21 and Skp2/p27[J]. Phytomedicine, 2022, 98: 153933.
[5]. Collado M, Blasco M A, Serrano M. Cellular senescence in cancer and aging[J]. Cell, 2007, 130(2): 223-233.
[6]. Mark A. Tirmenstein, Catherine X. Hu, Tracy L. Gales, Beverly E. Maleeff, Padma K. Narayanan, Edit Kurali, Timothy K. Hart, Heath C. Thomas, Lester W. Schwartz, Effects of Troglitazone on HepG2 Viability and Mitochondrial Function, Toxicological Sciences, Volume 69, Issue 1, September 2002, Pages 131–138, https://doi.org/10.1093/toxsci/69.1.131
[7]. Mikule K, Delaval B, Kaldis P, et al. Loss of centrosome integrity induces p38—p53—p21-dependent G1—S arrest[J]. Nature cell biology, 2007, 9(2): 160-170.
[8]. Shi, M.D., Lin, H.H., Lee, Y.C., Chao, J.K., Lin, R.A., Chen, J.H., 2008. Inhibition of cellcycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem. Biol. Interact. 174, 201–210.









