1. Introduction
Prunus mume is a deciduous tree of the genus Prunus in the family Rosaceae, whose flowers bloom in the cold winter and early spring with an elegant fragrance that is both ornamental, cultural and medicinal. Aroma of Prunus mume is a complex mixture of volatile organic compounds (VOCs), including terpenoids, phenylpropanoid compounds, and fatty acid derivatives, which together give Prunus mume unique olfactory characteristics. By systematically combing the chemical basis, metabolic regulatory mechanisms and domestic and international advances in key genes of Prunus mume floral scent components, this paper aims to reveal the molecular basis of aroma of Prunus mume, and to provide a theoretical basis for the molecular breeding and floral scent regulation of Prunus mume, in order to enhance its ornamental value and potential application prospects.
2. The study of the chemical composition of Prunus mume
2.1. Progress of research on chemical components of Prunus mume
The study of plant aroma chemistry has progressed significantly in recent years with rapid advances in analytical techniques and increased understanding of plant-environment interactions. The number of identified volatile chemicals synthesized by various plants exceeds 1000 and is likely to grow as more plants are examined by new methods for detecting and analyzing often minute amounts ofvolatiles [1, 2] .These volatile components can be mainly classified into terpenoids, phenylpropanoids and fatty acid derivatives. Knudsen et al. (1993) analyzed the aroma constituents of a variety of plants by gas chromatography-mass spectrometry (GC-MS) and found that these compounds usually have molecular weights lower than 300 and are highly lipid-soluble, are lipophilic molecules with high vapor pressures , with a variety of ecological functions [3] .
In addition, the chemical composition of plant aroma compounds is influenced by plant species, growing environment, and developmental stage [2]. Advancing analytical techniques have enabled researchers to gain a more comprehensive understanding of the chemical composition of plant aroma compounds and their role in plant physiological and ecological processes. Gas chromatography-mass spectrometry (GC-MS) is the main tool for analyzing plant aroma compounds with increasing resolution and sensitivity [3] . In addition, the application of mass spectrometry imaging (MSI) and high-resolution mass spectrometry (HRMS) has provided higher precision and spatial resolution for the analysis of plant aroma compounds [3].
Significant progress has been made in the study of plant aroma compounds, but there are still gaps in the research [2-4]. Among them, the regulation mechanism of biosynthesis of plant aroma compounds is not fully understood, especially under different environmental stress conditions [5-7] . On the other hand, the role of plant aroma compounds in plant-microbe interactions also needs to be further investigated [8] . Based on the current situation, future research on the chemical compounds of Prunus mume should focus on several aspects: researchers need to analyze the biosynthetic pathways of plant aroma compounds and their regulatory mechanisms, and at the same time pay attention to the specific functions of plant aroma compounds in plant-environment interactions [9,10] Researchers also need to develop new analytical techniques to improve the accuracy and efficiency of the detection of plant aroma compounds[11].
2.2. Progress of research on the chemical composition of aroma of Prunus mume
The formation of the aroma of Prunus mume characteristics stems from the systematic synthesis and synergistic action of its complex volatile components, in which the key regulation of phenylpropanoid and terpenoid metabolic pathways and the differences in the genetic background of varieties together shape the diversity and uniqueness of its aroma. Fewer international articles have been devoted to the study of aroma components released by Prunus mume. Some domestic studies have used headspace bagging or HS-SPME technology combined with GC-MS to identify the floral aroma components of some Prunus species, and concluded that the main volatiles include Benzyl acetate, benzyl alcohol, eugenol, methyl eugenol, benzaldehyde, and cinnamyl alcohol, etc., of which benzene ring/benzyl propane compounds are the most abundant and have the highest relative content. [12-15] However, the selected Prunus mum species were fewer, and the analyses were not sufficiently detailed. However, these studies selected fewer species of Prunus mume, and the analysis was not in-depth enough for further research. These aroma of Prunus mume compounds are mainly synthesized through the synergistic synthesis of phenylpropanoid pathway (Shikimic Acid pathway) and terpene metabolism pathway (Mevalonate pathway/MVA, Methyl Erythritol Phosphate pathway/MEP) [13, 16] . The synergistic interaction between different compounds constitutes the unique aroma profile of Prunus mume. Benzyl alcohol and benzaldehyde, as the main products of the phenylpropanoid pathway, present fresh and fruity aroma and bitter almond aroma, respectively, which are high in the aroma of Prunus mume and play a key role in the formation of the aroma of Prunus mume [14, 17]. In terpenoids, α-pinene's piney fragrance and linalool's sweet floral aroma enhanced the layers of aroma through the compound effect [13,18] .
In recent studies, scholars have found significant differences in the aroma composition of different varieties of Prunus mume [13, 15, 16]: some varieties have a strong aroma with eugenol and hexyl acetate as the main components, while other varieties have a clear aroma with benzyl alcohol and benzaldehyde as the main components, and the presence of important common compounds, such as hexyl acetate, eugenol, Benzyl acetate, and α-pinene, is the main reason for the similarity of aroma among the varieties of Prunus mume. The presence of important common compounds such as hexyl acetate, eugenol and α-pinene is the main reason for the similarity of the floral scent. Yang Yu et al. found that the cinnabar and palace powder variety groups had the highest number of floral aroma compounds and the highest number of phenyl ring/phenylpropane analogs, followed by the hopping variety group, the green calyx variety group, the jade butterfly variety group, and the drooping branch variety group [16]. This difference is mainly due to the differences in the types and contents of aroma substances contained in different varieties of Prunus mume, while the underlying reason may be the regulation of the biosynthetic pathways of floral aroma metabolism by the genetic background of different varieties of Prunus mume.
3. Biosynthetic pathways of floral scent metabolism in Prunus mume
3.1. Synthesis of terpenoids and phenylpropanoids
3.1.1. Domestic and international studies on the metabolic mechanisms of volatile organic compounds (VOCs) in plants
Research on the mechanism of plant VOC metabolism began to emerge in the 1990s, and international researchers have paid more attention to the mechanism of plant metabolomics, especially the research on the regulation of the synthesis of terpenoids and phenylpropanoid compounds. international theoretical studies on the metabolic pathways of floral aroma originated from the theoretical model of mevalonate (MVA) pathway established by Chappell (1995), and the discovery of methylerythritol phosphate (MEP) pathway by Lichtenthaler (1997). These seminal works provided key methodological support for subsequent studies. In 2005, Dudareva et al. further found that the synthesis of monoterpenes and sesquiterpenes in Lonchura stramonium is mainly accomplished via the MEP pathway, while the MVA pathway provides additional precursors in some cases [19] . Recently, Dudareva's team revealed the mechanism of carbon competition through metabolic flux analysis and found that the synthesis of sesquiterpenes was reduced by 23% when the MEP pathway was active, a breakthrough that pushes the research to the level of dynamic balance of metabolic networks [11, 20] . It is worth noting that, in response to the academic controversy between the "hormone-dominant theory" (JA signaling core theory) and the "environment-responsive theory" (light-temperature synergism theory) of terpene synthesis regulation mechanism, studies in support of the "hormone-dominant theory" have found that jasmonic acid (JA) plays a central role in terpene synthesis. [21] Hong et al. (2012) found in Arabidopsis thaliana inflorescences that the bHLH transcription factor MYC2 activates the expression of two sesquiterpene synthase genes, TPS11 and TPS21, through the GA (Gibberellic Acid) and JA (Jasmonic Acid) signaling pathways, thereby regulating terpene synthesis [22] . This finding suggests a key role for endogenous hormone signaling in the regulation of terpene synthesis. In 2008, Kessler's research team further confirmed the importance of endogenous hormones in the regulation of terpene synthesis by studying the interaction between tobacco and cotton bollworms and finding that the JA signaling pathway plays a dominant role in inducing the release of volatile terpenoids in plants in response to insect feeding [23] . The influence of environmental factors on terpene synthesis has also been emphasized by international researchers on the one hand in the "environmental response theory". In 2009, a team of Cordoba researchers found that light, as an important environmental signal, significantly affects the expression of MEP pathway genes and terpene synthesis in plants. They noted that light has been shown to activate specific transcription factors such as members of the PIFs family, which may indirectly regulate the expression of the DXS (1-deoxy-D-xylulose-5-phosphate synthase) gene. DXS, a key enzyme in the MEP pathway, has been shown to have a direct effect on terpene synthesis by its activity[19] . In addition, temperature also has a great effect on terpene synthesis. Further studies have shown that the synthesis of certain terpenoids in plants is inhibited at low temperatures, while high temperatures may promote their synthesis, suggesting that temperature in synergy with light may further regulate terpenoid synthesis by affecting physiological states and metabolic pathways in plants[24] . Existing international research also suggests that the biosynthesis of plant aroma compounds also involves the participation of various enzymes including terpene synthase (TPS) and cytochrome P450 enzymes (P450) [25, 26] .
Domestic research on the aroma metabolism of Prunus mume began with the development of characteristic plant resources under the guidance of the strategy of modernization of traditional Chinese medicine. With the implementation of the Special Plan for Breeding Specialty Ornamental Plants, Fei et al. revealed in 2010 that activation of the benzyl benzoate synthesis pathway and inhibition of the benzyl alcohol synthesis pathway were the two main causes of the lack of floral scent in Prunus apricot varieties through metabolism, enzyme activity, and transcriptomics analyses [27] . Prof. Chen's research team revealed high-resolution cellular profiles of petals at different stages of flowering and development of Prunus mume by single-cell RNA sequencing analysis in 2024, which pushed the metabolic mechanism of Prunus mume into the stage of temporal and spatial dynamics analysis in China [28]. However, the existing results in China are mostly focused on the analysis of metabolic pathways, but still lag behind the international advanced level in the study of key enzymes post-translational modification, metabolite transmembrane transport and other in-depth mechanisms, especially in the translation of the results, Chen Qingshuai led the research team to obtain PmMYB1 overexpression strain (aroma enhancement of 2.5-fold), but the lack of field stability of the strain may be a certain constraint in the dissemination of this strain in the follow-up of the industrial application of the strain. The lack of field stability may be a constraint in the subsequent industrialization of this strain.[29]
3.1.2. Progress in terpenoids and phenylpropanoids research
Terpenoids and phenylpropanoids act as central components in the synthesis pathway of volatile organic compounds (VOCs) that give Prunus mume their unique and complex aroma. Terpenoids can account for 50% to 70% of the volatile constituents in Prunus mume, and the biosynthesis of terpenoids relies on the mevalonate (MVA) pathway in the cytoplasm and the methylerythritol phosphate (MEP) pathway in the plastid.
The key rate-limiting enzyme of the MVA pathway, HMGR (3-hydroxy-3-methylglutaryl coenzyme A reductase), catalyzes the conversion of HMG-CoA to MVA, and the expression level of this enzyme is significantly and positively correlated with the accumulation of sesquiterpenes [30] . Thereafter, HMG-CoA was converted to isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP) through a series of phosphorylation reactions, and formed farnesyl pyrophosphate under the action of FPPS (farnesyl pyrophosphate synthase), and the above findings laid the foundation for the direction of the biosynthesis research of sesquiterpenes [31] . Subsequent findings in this part of the work have also illustrated that bZIP and WRKY transcription factors may regulate their expression by binding to the HMGR promoter, and that jasmonic acid (JA) has been shown to induce the activity of HMGR and thus promote the synthesis of sesquiterpenes [20] .
The MEP pathway occurs mainly within the plastid, and the rate-limiting enzyme DXS (1-deoxy-D-xylulose-5-phosphate synthase) may directly determine the synthesis flux of monoterpenes. In Plumeria petals, expression of PmDXS was verified to be significantly higher than that in leaves and positively correlated with linalool accumulation by three replicate biological validations [8, 32] . Further monoterpenes were efficiently synthesized under the catalytic action of GPPS (geranylgeranyl pyrophosphate synthase) and TPS (terpene synthase). Light signaling and epigenetic modifications (e.g. DNA demethylation) have also been shown to be important regulators of PmDXS expression in Prunus [24, 29 , 33] .
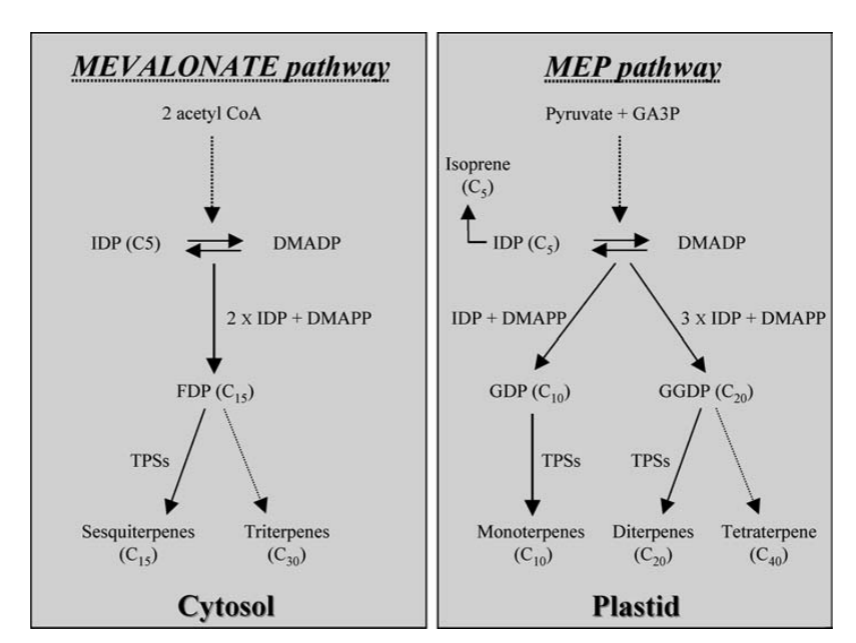
Figure 1: Metabolic engineering of terpenoid biosynthesis in plants [34]
Unlike terpenoids, phenylpropanoids are used as the main source of the sweet the aroma of Prunus mume. The biosynthesis of phenylpropanoids has been elucidated: it starts with phenylalanine and is catalyzed by phenylalanine ammonia lyase (PAL) to cinnamic acid, which is then converted to phenylacetaldehyde in a series of enzymatic reactions to phenylethanol by phenylacetaldehyde reductase (PAR), thus becoming an important component of the aroma of Prunus mume [35, 36] . In this biosynthetic pathway, PAAS (phenylacetaldehyde synthase) and PAR are responsible for the intermediate conversion and final reduction, respectively. A team of researchers has demonstrated that the expression level of PmPAL is positively correlated with phenylethanol accumulation, and that PmMYB1, a MYB transcription factor, promotes the expression of PAL and PAR genes by binding to their promoter regions to increase the phenylethanol content of transgenic Prunus mume by 2.5-fold [21, 37, 38] . Environmental factors also have an important effect on the synthesis process, and low temperature (4°C) as the main trigger induced a 30%-50% increase in phenylethanol accumulation by PmPAL expression, a phenomenon that affects the aromatic properties of Prunus mume and to a certain extent proves to be associated with cold tolerance [39] . The spatial distribution of phenylpropanoid metabolism also shows a clear subcellular division of labor, with the early steps taking place mainly in the cytoplasm, including those catalyzed by phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), and 4-coumarate coenzyme A ligase (4CL), and the subsequent steps (e.g., PAR) in peroxisomes, which together allow the cell to avoid toxicity caused by the intermediary products of metabolism. Products [10] .
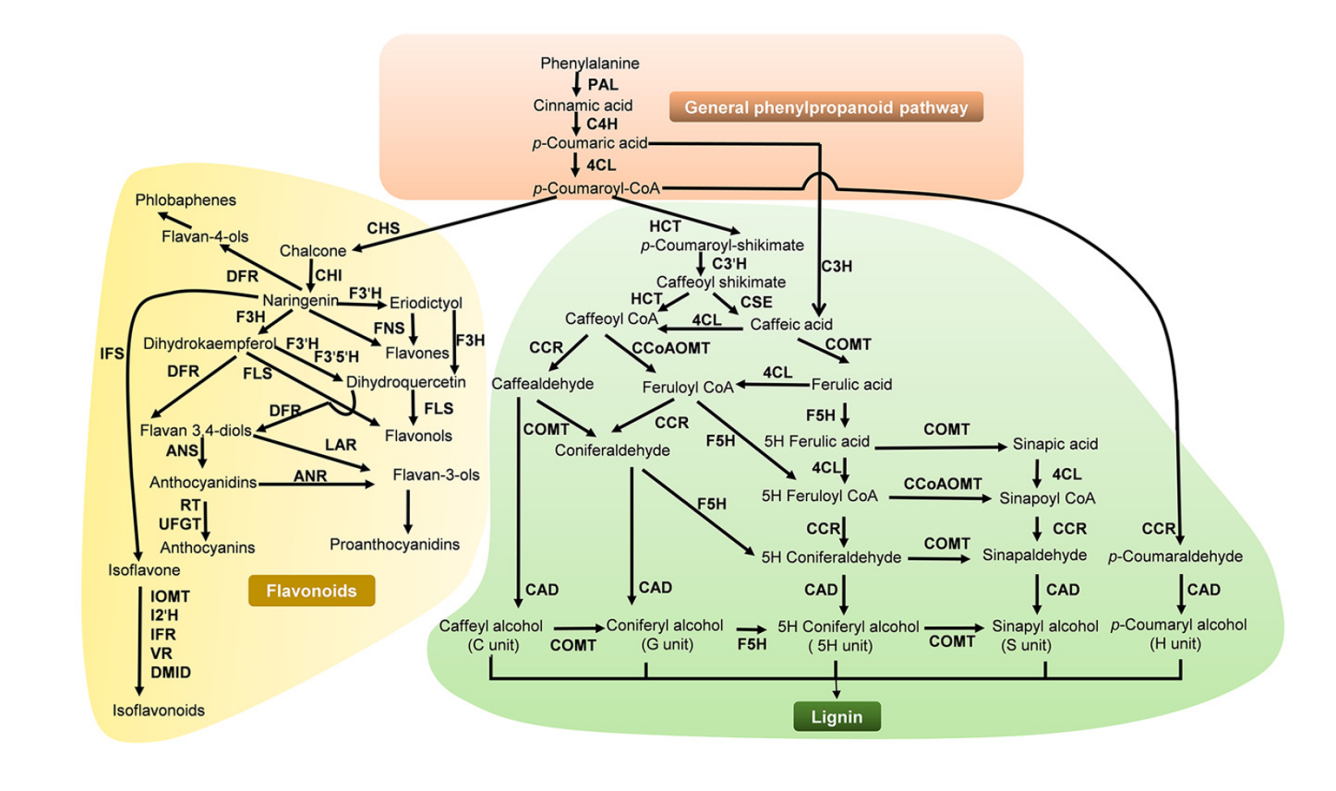
Figure 2: Complex mechanisms controlling phenylpropane metabolite biosynthesis and regulation of the phenylpropane pathway [10]
3.2. Cross-regulation of synthetic pathways
It is worth noting that the synthesis of terpenoids and phenylpropanoid compounds in Prunus mume is not carried out in isolation, but through the sharing of precursors (e.g., acetyl-coenzyme A) as well as regulatory factors, thus realizing a dynamic equilibrium. Under the carbon source competition mechanism, the activity of the MEP pathway directly affects the supply of pyruvate in the plastid, which in turn weakens the utilization of acetyl coenzyme A by the MVA pathway, which influences the synthesis of sesquiterpenes [20] . Hormonal regulation, as another aspect, also plays an important role in this process as well: jasmonic acid (JA) not only induces the expression of PmDXS and promotes terpenoid synthesis but also activates PmMYB1 to enhance the synthesis of phenylpropanoid compounds. However, gibberellin (GA) exhibited the effect of inhibiting PAL activity to suppress the synthesis of phenylpropanoids [29] . Overall, the metabolic pathways of terpenoids and phenylpropanoids have a complex interplay, and this dynamic regulatory mechanism enables a more sophisticated aroma formation program in Prunus mume, providing a new research direction for future Prunus mume varietal improvement and aroma regulation.
Existing future researchers should incorporate new research pathways and methods to further clarify the mechanism of the biosynthetic pathway of Prunus mume floral metabolism, e.g., researchers can focus on the development of CRISPR-Cas12i, a novel metabolic pathway precision editing technology, to realize the precise regulation of metabolic pathways [40] . Researchers can also visualize the spatial distribution of metabolic processes by constructing 3D visual models of petal metabolic microregions, a new research technique [41] . Finally, researchers can analyze the ecological interaction network of aroma volatiles to reveal the role of aroma substances in the ecosystem. These three discoveries can establish the research framework of "metabolic module-regulatory node-environmental response", which is expected to break through the technical bottleneck in the directional regulation of the aroma of Prunus mume, and provide new ideas and methods for the research and application in related fields.
4. Key genes in the regulation of the aroma of Prunus mume
4.1. Genes related to synthesis of aromatic components
In recent years, with the rapid development of genome sequencing technology, related genomics and other histological studies have been widely used by researchers, and the key gene regulatory networks of plant fragrance have been gradually analyzed by domestic and international researchers. Currently, several key genes have been identified to regulate the aroma biosynthesis of Prunus mume, including genes for aroma synthases, transcription factors, and transporter proteins. These genes can affect the synthesis, transportation and release of the fragrance of Prunus mume through a complex regulatory network. Specifically, the expansion of the PmBEAT gene family in Prunus mume has been suggested to be a key factor in the formation of its distinctive floral scent. Fei et al. showed that overexpression of the PmBEAT36 or PmBEAT37 genes increased the production of benzyl acetate in the protoplasts of Prunus mume petals, thereby inducing the production of Prunus mume's characteristic oolong aroma. [42] . Tengxun et al. found that Tengxun et al. found that the PmCFAT1 gene is involved in the in vitro biosynthesis of eugenol in Prunus mume by promoting the conversion of eugenol to eugenol catalyzed by eugenol synthase (EGS) [39] . The expression of these genes is precisely regulated by a synergistic combination of transcription factors and hormonal signals, which play a key role in the aroma of Prunus mume formation. On the other hand, recent studies have revealed that PmBAHD3, PmBAHD5 and PmEGS1 are involved in the formation of floral aroma volatiles through their expression in thin-walled and epidermal cells associated with floral aroma synthesis in Prunus mume petals, where PmBAHD5 (a direct homolog of PmBEAT36) catalyzes the generation of benzyl acetate from benzyl alcohol, and PmBAHD3 may be involved in the synthesis of benzyl acetate and eugenol synthesis [28] . Wang et al. By analogy with genes with proven functions in Arabidopsis thaliana, the predicted three PmCAD genes, two 4CL genes, three CCR genes, and two IGS genes contributed significantly to the synthesis of both benzyl acetate and eugenol in Qingdao Prunus mume cultivars, and the downstream genes, on the other hand, included PmBGLU 18-like, PmUGT71A16 and PmUGT73C6 are collectively involved in the matrix binding of the aroma of Prunus mume substances as well as the regulation of volatile state [43] .
4.2. Transcription factor regulation
Transcription factors also play important roles in the regulation of floral scent of Prunus mume. a research team of Wang et al. compared and analyzed the transcriptomes of different Prunus mume varieties at different flowering stages and found that PmPAL and PmMYB4_TF may play prominent roles in regulating the accumulation of key precursor substrates (trans-cinnamic acid and p-coumaric acid derivatives) for the main aroma compounds of Prunus mume, possibly by modulating the expression of key genes in the mangiferic acid metabolic pathway. play prominent roles. Among them, PmMYB4 transcription factor was significantly up-regulated in Prunus and associated with phenolic compound metabolism and phenylpropanoid biosynthesis [43] , while PmPAL, the first and key enzyme in phenylpropanoid metabolism sequence, not only plays a role in the regulation of Prunus mume flower aroma by increasing the accumulation of phenylethanol, but also is associated with the regulation of Prunus mume cold tolerance [6] . The discovery of these transcription factors provides important clues to reveal the molecular regulatory mechanism of Prunus mume fragrance formation. International research is still focused on the basic discovery stage, and although the number of research results has been on the rise in recent years, the depth of research needs to be further expanded.
4.3. Genes and hormonal influences on the transportation of aromatic components of Prunus mume
In addition to the synthesis of aromatic components, their intracellular transport is also a key component of floral fragrance release. Studies have shown that PmABCG9, an ABCG family protein that specifically transports benzyl alcohol, exists in Prunus mume[44] PmABCG9 has been predicted to have a typical NBD-TMD structural domain, belongs to the WBC type of the ABCG subfamily, and encodes an ATP-type binding cassette (ABC) transporter protein [45] . Meanwhile, subcellular localization results showed that PmABCG9 was expressed on the cell membrane, suggesting that it could efficiently transport aromatic components, such as benzyl alcohol, to the extracellular compartments in order to increase the volatilization of aromatic components [44] .
Hormone signaling, consistent with the above, also plays an important regulatory role in the synthesis and transport of aromatic components in Prunus mume. Methyl jasmonate (MeJA), an important phytohormone, significantly affects the synthesis and volatilization of aromatic components such as benzyl alcohol in Prunus mume, and MeJA promotes the synthesis of benzyl alcohol through the activation of genes such as PmABCG9 and accelerates the transmembrane transport of aromatic components by regulating the activity of transporter proteins such as ABC. In line with the above, gibberellins (GA) exhibit an inhibitory effect on the activity of PmPAL and inhibit the synthesis of phenylpropanoid compounds [6] . Phytohormones such as growth hormone and ethylene may also indirectly regulate the synthesis and transport of aromatic components by affecting cellular metabolism and gene expression.
5. Conclusion
Comprehensive domestic and international research progress on the aroma components of Prunus mume and its regulatory mechanism, domestic scholars have achieved remarkable results in the fields of chemical composition, metabolic pathway analysis and key gene function verification of the aroma components of Prunus mume. Focusing on the synergistic effect of phenylpropanoid and terpene metabolic pathways, the study systematically revealed the expression pattern of key genes, such as PmPAL and PmDXS, and their interactions with environmental factors (e.g., low temperatures) through metabolomics and transcriptomics technologies. The domestic team innovatively discovered that PmMYB4 transcription factor can activate the phenylpropanoid metabolic pathway to increase phenylethanol content by 2.5 times, which provides an important target for molecular breeding. Meanwhile, the research focuses on application-oriented, developed new technologies such as single-cell metabolomics, and promoted the translation of theoretical research to the field. However, there are still shortcomings in the analysis of metabolite transmembrane transport, post-translational modification of key enzymes, and the field stability of metabolically engineered strains (<60%) needs to be improved.
Overseas studies focused on the construction of basic theories of plant aroma metabolism and made breakthroughs in the areas of the carbon source competition mechanism of the MEP/MVA pathway, hormone signaling network (such as the antagonistic regulation of JA-GA) and epigenetic modification. The scholars quantified the inhibitory effect of MEP pathway activity on sesquiterpene synthesis (23%) by metabolic flux analysis and constructed a dynamic metabolic model including light-temperature responsive components. It is worth noting that international research still focuses on model plants (e.g., Arabidopsis thaliana and tobacco), and the research on specific subclasses of Prunus species is not in-depth and lacks specific applications.
Current research shows a clear trend of cross-disciplinary but still needs to be deepened in the following aspects: existing articles in the epigenetic modification of TPS isomer selectivity has not been elucidated in the regulatory mechanism of the depth of the mechanism of research has limited the understanding of the spatio-temporal specificity of the formation of the aroma. Part of the research relies on in vitro enzyme activity detection, and the lack of in vivo metabolic imaging technology makes it difficult to capture the real metabolic process in vivo, and most of the research focuses on the enhancement of the ornamental value, and there is insufficient exploration of ecological interactions, medicinal components development and other extended areas. Future research should focus on building a research framework of "metabolic module-regulatory node-environmental response": technological innovation needs to focus on the development of CRISPR-Cas12i-mediated metabolic pathway editing technology, combined with the petal metabolism microcellular 3D visualization model, to achieve dynamic analysis of aroma synthesis process (40, 41). The authors of this paper still recommend that scholars should take a look at the current stage. The authors of this paper still suggest that scholars should focus on the screening of storage and transportation tolerance gene modules through the integrated multi-omics analysis, so as to cultivate new varieties with both aroma stability and environmental adaptability at this stage.
By systematically analyzing the molecular basis of the aroma metabolism of Prunus mume, this study provides a new strategy for the improvement of the quality of traditional flowers, as well as a theoretical reference for the transformation of the secondary metabolism of plants towards the construction of multilevel regulatory networks. This paper suggests that scholars can explore the synergistic effects of signaling pathways such as jasmonic acid and epigenetic modifications in the subsequent mechanistic studies by strengthening cross-species comparative analyses to explore the unique trait formation mechanism of the aroma metabolism of Prunus mume, to develop a new pathway for the study of the directional control of the aroma of Prunus mume.
References
[1]. Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol ,20025 ( 3), 237-243. DOI: https://doi.org/10.1016/S1369-5266(02)00251-0 From NLM
[2]. Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol ,2004135 (4), 1893 1902. DOI: https://doi.org/10.1104/ pp.104.049981 From NLM.
[3]. Knudsen, J. T.; Tollsten, L.; Bergström, L. G. Floral scents-a checklist of volatile compounds isolated by head space techniques. Phytochemistry ,199333 (2), 253-280. DOI: https://doi.org/10.1016/0031-9422(93)85502-I .
[4]. Pichersky, E.; Noel, J. P.; Dudareva, N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science ,2006311 (5762), 808-811. DOI: https:/ /doi.org/10.1126/science.1118510 From NLM.
[5]. D'Auria, J. C.; Chen, F.; Pichersky, E. Characterization of an acyltransferase capable of synthesizing benzylbenzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol ,2002130 (1), 466-476. DOI: 10.1104/pp.006460 From NLM.
[6]. Li, P.; Zheng, T.; Li, L.; Liu, W.; Qiu, L.; Ahmad, S.; Wang, J.; Cheng, T.; Zhang, Q. Integration of chromatin accessibility and gene expression reveals new regulators of cold hardening to enhance freezing tolerance in Prunus mume. J Exp Bot ,202374 (6), 2173-2187. DOI: https://doi.org/10.1093/jxb/ erad027 From NLM.
[7]. Li, S. J.; Liu, S. C.; Lin, X. H.; Grierson, D.; Yin, X. R.; Chen, K. S. Citrus heat shock transcription factor CitHsfA7-mediated citric acid degradation in response to heat stress. Plant Cell Environ ,202245 (1), 95-104. DOI: 10.1111/pce.14207 From NLM。
[8]. Zhang, Y.; Wang, J.; Cao, X.; Liu, W.; Yu, H.; Ye, L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzyme Microb Technol ,2020134 , 109462. DOI: https://doi.org/10.1016/j.enzmictec.2019.109462 From NLM
[9]. Turlings, T. C.; Tumlinson, J. H.; Lewis, W. J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science ,1990250 (4985), 1251-1253. DOI: 10.1126/science.250.4985.1251 From NLM.
[10]. Dong, N. Q.; Lin, H. X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J Integr Plant Biol ,202163 ( 1), 180-209. DOI: https://doi.org/10.1111/jipb.13054 From NLM.
[11]. Dudareva, N.; DellaPenna, D. Plant metabolic engineering: future prospects and challenges. Curr Opin Biotechnol,201324 (2), 226-228. DOI: https://doi .org/10.1016/j.copbio.2013.02.002 From NLM.
[12]. Wang Liping, Liu. Wang Li-Ping, Liu. A preliminary study on the aroma composition of Prunus mume. Journal of Horticulture2003 , (01), 42. DOI: https://doi.org/10.16420/j.issn.0513-353x.2003.01.010 From Cnki.
[13]. Jin Hexian; Chen Junyu; Jin Youju. Comparative study on the aroma composition of different types of Prunus mume varieties in Nanjing. Journal of Horticulture2005 , (06), 1139. DOI: https://doi.org/10.16420/j.issn.0513 353x.2005.06.044 From Cnki.
[14]. Cao, Hui; Li, Zuguang; Wang, Yan; Shen, Delong. Analysis and QSRR study of the aroma components of two species of Prunus mume. Journal of Analytical Science ,200925 (02), 130-134. From Cnki.
[15]. Zhao, Yinquan; Pan, Huitang; Zhang, Qixiang; Sun, Ming; Pan, Caibo. Studies on the volatile components of different types of Prunus species. Journal of Tropical Subtropical Botany ,201018 (03), 310-315. From Cnki.
[16]. Yang Yu; Wang Yiguang; Dong Bin; Xiao Zheng; Zhao Hongbo. Identification and analysis of floral scent components of different Prunus species. Journal of Zhejiang Agriculture and Forestry University ,202441 (02), 262274. From Cnki.
[17]. Wang Shuang; Dong Bin; Wang Yiguang; Zhao Hongbo. Analysis and evaluation of fruit and flower characteristics of different plum varieties. Journal of Zhejiang Agriculture and Forestry University ,202441 (01), 113-123. From Cnki.
[18]. Zhong Chunlin; He Jiaying; Qiu Yunjing; Lin Han; Pan Liye; Liang Yongxin; Li Guowei; Lan Xiaoyong; Chen Xiangdong. UPLC Characterization and Determination of Flavonoid Content of Wax Myrtle Flower Herbs. Journal of Guangdong Pharmaceutical University ,202339 (06), 65-69. DOI: https://doi.org/10.16809/j.cnki.2096 3653.2023073101 From Cnki.
[19]. Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci U S A ,2005102 (3), 933-938. DOI: https://doi.org/10.1073/pnas.0407360102 From NLM
[20]. Dudareva, N.; Klempien, A.; Muhlemann, J. K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol ,2013198 (1), 16-32. DOI: https://doi.org/10.1111/nph.12145 From NLM.
[21]. Muhlemann, J. K.; Klempien, A.; Dudareva, N. Floral volatiles: from biosynthesis to function. Plant Cell Environ ,201437 (8), 1936-1949. DOI: https:// doi.org/10.1111/pce.12314 From NLM.
[22]. Hong, G. J.; Xue, X. Y.; Mao, Y. B.; Wang, L. J.; Chen, X. Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. expression. Plant Cell ,201224 (6), 2635-2648. DOI: https://doi.org/10.1105/tpc.112.098749 From NLM.
[23]. Kessler, D.; Gase, K.; Baldwin, I. T. Field experiments with transformed plants reveal the sense of floral scents. Science ,2008321 (5893), 1200-1202. DOI. https://doi.org/10.1126/science.1160072 From NLM.
[24]. Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol ,201364 , 665-700. DOI: https ://doi.org/10.1146/annurev-arplant-050312-120116 From NLM.
[25]. Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A ,199895 (8),. 4126-4133. DOI: https://doi.org/10.1073/pnas.95.8.4126 From NLM.
[26]. Lupien, S.; Karp, F.; Wildung, M.; Croteau, R. Regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha) species: cDNA isolation, characterization, and functional expression of (-)-4S-limonene-3-hydroxylase and (-)-4S-limonene-6-hydroxylase. Arch Biochem Biophysics , () characterization, and functional expression of (-)-4S-limonene-3-hydroxylase and (-)-4S-limonene-6-hydroxylase. Arch Biochem Biophys ,1999368 (1), 181-192. DOI: https://doi.org/10.1006/abbi.1999.1298 From NL
[27]. Bao, F.; Zhang, T.; Ding, A.; Ding, A.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Metabolic, Enzymatic Activity, and Transcriptomic Analysis Reveals the Mechanism Underlying the Lack of Characteristic Floral Scent in Apricot Mei Varieties. Front Plant Sci ,202011 , 574982. DOI: https://doi.org/10.3389/ fpls.2020.574982 From NLM
[28]. https://doi.org/10.1093/hr/uhae189From NLM.[29]Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends in Plant Science ,202227 (4), 379-390. DOI: https. //doi.org/10.1016/j.tplants.2021.10.009 .[30]Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the Reaction Catalyzed by 3-Hydroxy-3Methylglutaryl Coenzyme A Reductase a Rate- Limiting Step for Isoprenoid Biosynthesis in Plants? Plant Physiol ,1995109 (4), 1337-1343. DOI:
[29]. Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends in Plant Science ,202227 (4), 379-390. DOI: https. //doi.org/10.1016/j.tplants.2021.10.009 .
[30]. Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the Reaction Catalyzed by 3-Hydroxy-3 Methylglutaryl Coenzyme A Reductase a Rate- Limiting Step for Isoprenoid Biosynthesis in Plants? Plant Physiol ,1995109 (4), 1337-1343. DOI: https://doi.org/10.1104/pp.109.4.1337 From NLM.
[31]. Basak, A.; Chakrabarty, K.; Ghosh, A.; Das, G. K. DFT study on the mechanism of 1,3-hydrogen disposition in Isopentenyl pyrophosphate catalyzed by Isopentenyl pyrophosphate: Dimethylallyl pyrophosphate isomerase. Journal of Theoretical and Computational Chemistry ,201615 (03), 1650025. DOI: https. //doi.org/10.1142/s0219633616500255 .
[32]. Lichtenthaler, H. K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate- independent pathway. FEBS Lett ,1997400 (3), 271-274. DOI: https://doi.org/10.1016/S0014-5793(96)01404-4 From NLM.
[33]. Hu, Y.; Zheng, T.; Dong, J.; Li, W.; Ma, X.; Li, J.; Fang, Y.; Chen, K.; Zhang, K. Regulation of the main terpenoids biosynthesis and accumulation in Horticultural Plant Journal2024 . DOI: https://doi.org/10.1016/j.hpj.2024.08.002。
[34]. Aharoni, A.; Jongsma, M. A.; Kim, T.-Y.; Ri, M.-B.; Giri, A. P.; Verstappen, F. W. A.; Schwab, W.; Bouwmeester, H. J. Metabolic Engineering of Terpenoid Biosynthesis in Plants. Phytochemistry Reviews ,20065 (1), 49-58. DOI: https://doi.org/10.1007/s11101-005-3747-3 .
[35]. Boatright, J.; Negre, F.; Chen, X.; Kish, C. M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in Vivo Benzenoid Metabolism in Petunia Petal Tissue. Plant Physiology ,2004135 (4), 1993-2011. DOI: https://doi.org/10.1104/pp.104.045468 (accessed 4/5/2025).
[36]. Chen, R.; Yang, S.; Zhang, L.; Zhou, Y. J. Advanced Strategies for Production of Natural Products in Yeast. iScience ,202023 (3), 100879. DOI: https://doi. org/10.1016/j.isci.2020.100879 .
[37]. Kaminaga, Y.; Schnepp, J.; Peel, G.; Kish, C. M.; Ben-Nissan, G.; Weiss, D.; Orlova, I.; Lavie, O.; Rhodes, D.; Wood, K.; et al. Plant Phenylacetaldehyde Synthase Is a Bifunctional Homotetrameric Enzyme That Catalyzes Phenylalanine Decarboxylation and Oxidation*. Journal of Biological Chemistry ,2006281 (33), 23357-23366. DOI: https://doi.org/10.1074/jbc.M602708200 .
[38]. Wang, X.; Song, Z.; Ti, Y.; Liu, Y.; Li, Q. Physiological response and transcriptome analysis of Prunus mume to early salt stress. Journal of Plant Journal of Plant Biochemistry and Biotechnology ,202231 (2), 330-342. DOI: https://doi.org/10.1007/s13562-021-00680-2 .
[39]. Zhang, T.; Huo, T.; Ding, A.; Hao, R.; Wang, J.; Cheng, T.; Bao, F.; Zhang, Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS One , (10), e022397 activity analysis of coniferyl alcoholacetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS One ,201914 (10), e0223974. DOI: https. //doi.org/10.1371/journal.pone.0223974 From NLM.
[40]. Chen, Qiu-Chong; Li, Shang-Pu; Miao, Er-Yu; Zhou, Bing-Qian; Wang, X.; Meng, Xiang-Yu; Wang, Xiao-Long; Xu, Kun. HDACi and RS-1 improve the efficiency of CRISPR/Cas12i-mediated HDR editing. Journal of Agricultural Biotechnology ,202432 (10), 2306-2323. from Cnki.
[41]. Ma, X.; Shedlock, C. J.; Medina, T.; Ribas, R. A.; Clarke, H. A.; Hawkinson, T. R.; Dande, P. K.; Wu, L.; Burke, S. N.; Merritt, M. E.; et al. MetaVision3D : Automated Framework for the Generation of Spatial Metabolome Atlas in 3D. bioRxiv2023 . DOI: https://doi.org/10.1101/2023.11.27.568931 From NLM.
[42]. Bao, F.; Ding, A.; Zhang, T.; Luo, L.; Wang, J.; Cheng, T.; Zhang, Q. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic Res ,20196 , 24. DOI: https://doi.org/10.1038/s41438-018-0104-4 From NLM.
[43]. Xiujun, W.; Zhenqi, S.; Yujing, T.; Kaifeng, M.; Qingwei, L. Comparative transcriptome analysis linked to key volatiles reveals molecular mechanisms of aroma compound biosynthesis in Prunus mume. BMC Plant Biol ,202222 (1), 395. DOI: https://doi.org/10.1186/s12870-022-03779-3 From NLM.
[44]. Hao, R. J.; Qiu, C.; Geng, X. Y.; Jia, H. T.; Zhang, Y. J.; Chang, J.; Feng, X. X.. Functional analysis of Plum Pm ABCG9 in benzyl alcohol volatilization. Chinese Agricultural Science ,202356 (13), 2574-2585. From Cnki.
[45]. Hao, R.; Yang, S.; Zhang, Z.; Zhang, Y.; Chang, J.; Qiu, C. Identification and specific expression patterns in flower organs of ABCG genes related to floral scent from Prunus mume. Scientia Horticulturae ,2021288 , 110218. DOI: https://doi.org/10.1016/j.scienta.2021.110218
Cite this article
Zhang,Y. (2025). Progress on the Chemical Basis, Metabolic Regulation Mechanism and Key Genes of the Floral Components of Prunus Mume. Theoretical and Natural Science,112,165-176.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICEGEE 2025 Symposium: Sensor Technology and Multimodal Data Analysis
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol ,20025 ( 3), 237-243. DOI: https://doi.org/10.1016/S1369-5266(02)00251-0 From NLM
[2]. Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol ,2004135 (4), 1893 1902. DOI: https://doi.org/10.1104/ pp.104.049981 From NLM.
[3]. Knudsen, J. T.; Tollsten, L.; Bergström, L. G. Floral scents-a checklist of volatile compounds isolated by head space techniques. Phytochemistry ,199333 (2), 253-280. DOI: https://doi.org/10.1016/0031-9422(93)85502-I .
[4]. Pichersky, E.; Noel, J. P.; Dudareva, N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science ,2006311 (5762), 808-811. DOI: https:/ /doi.org/10.1126/science.1118510 From NLM.
[5]. D'Auria, J. C.; Chen, F.; Pichersky, E. Characterization of an acyltransferase capable of synthesizing benzylbenzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol ,2002130 (1), 466-476. DOI: 10.1104/pp.006460 From NLM.
[6]. Li, P.; Zheng, T.; Li, L.; Liu, W.; Qiu, L.; Ahmad, S.; Wang, J.; Cheng, T.; Zhang, Q. Integration of chromatin accessibility and gene expression reveals new regulators of cold hardening to enhance freezing tolerance in Prunus mume. J Exp Bot ,202374 (6), 2173-2187. DOI: https://doi.org/10.1093/jxb/ erad027 From NLM.
[7]. Li, S. J.; Liu, S. C.; Lin, X. H.; Grierson, D.; Yin, X. R.; Chen, K. S. Citrus heat shock transcription factor CitHsfA7-mediated citric acid degradation in response to heat stress. Plant Cell Environ ,202245 (1), 95-104. DOI: 10.1111/pce.14207 From NLM。
[8]. Zhang, Y.; Wang, J.; Cao, X.; Liu, W.; Yu, H.; Ye, L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzyme Microb Technol ,2020134 , 109462. DOI: https://doi.org/10.1016/j.enzmictec.2019.109462 From NLM
[9]. Turlings, T. C.; Tumlinson, J. H.; Lewis, W. J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science ,1990250 (4985), 1251-1253. DOI: 10.1126/science.250.4985.1251 From NLM.
[10]. Dong, N. Q.; Lin, H. X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J Integr Plant Biol ,202163 ( 1), 180-209. DOI: https://doi.org/10.1111/jipb.13054 From NLM.
[11]. Dudareva, N.; DellaPenna, D. Plant metabolic engineering: future prospects and challenges. Curr Opin Biotechnol,201324 (2), 226-228. DOI: https://doi .org/10.1016/j.copbio.2013.02.002 From NLM.
[12]. Wang Liping, Liu. Wang Li-Ping, Liu. A preliminary study on the aroma composition of Prunus mume. Journal of Horticulture2003 , (01), 42. DOI: https://doi.org/10.16420/j.issn.0513-353x.2003.01.010 From Cnki.
[13]. Jin Hexian; Chen Junyu; Jin Youju. Comparative study on the aroma composition of different types of Prunus mume varieties in Nanjing. Journal of Horticulture2005 , (06), 1139. DOI: https://doi.org/10.16420/j.issn.0513 353x.2005.06.044 From Cnki.
[14]. Cao, Hui; Li, Zuguang; Wang, Yan; Shen, Delong. Analysis and QSRR study of the aroma components of two species of Prunus mume. Journal of Analytical Science ,200925 (02), 130-134. From Cnki.
[15]. Zhao, Yinquan; Pan, Huitang; Zhang, Qixiang; Sun, Ming; Pan, Caibo. Studies on the volatile components of different types of Prunus species. Journal of Tropical Subtropical Botany ,201018 (03), 310-315. From Cnki.
[16]. Yang Yu; Wang Yiguang; Dong Bin; Xiao Zheng; Zhao Hongbo. Identification and analysis of floral scent components of different Prunus species. Journal of Zhejiang Agriculture and Forestry University ,202441 (02), 262274. From Cnki.
[17]. Wang Shuang; Dong Bin; Wang Yiguang; Zhao Hongbo. Analysis and evaluation of fruit and flower characteristics of different plum varieties. Journal of Zhejiang Agriculture and Forestry University ,202441 (01), 113-123. From Cnki.
[18]. Zhong Chunlin; He Jiaying; Qiu Yunjing; Lin Han; Pan Liye; Liang Yongxin; Li Guowei; Lan Xiaoyong; Chen Xiangdong. UPLC Characterization and Determination of Flavonoid Content of Wax Myrtle Flower Herbs. Journal of Guangdong Pharmaceutical University ,202339 (06), 65-69. DOI: https://doi.org/10.16809/j.cnki.2096 3653.2023073101 From Cnki.
[19]. Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci U S A ,2005102 (3), 933-938. DOI: https://doi.org/10.1073/pnas.0407360102 From NLM
[20]. Dudareva, N.; Klempien, A.; Muhlemann, J. K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol ,2013198 (1), 16-32. DOI: https://doi.org/10.1111/nph.12145 From NLM.
[21]. Muhlemann, J. K.; Klempien, A.; Dudareva, N. Floral volatiles: from biosynthesis to function. Plant Cell Environ ,201437 (8), 1936-1949. DOI: https:// doi.org/10.1111/pce.12314 From NLM.
[22]. Hong, G. J.; Xue, X. Y.; Mao, Y. B.; Wang, L. J.; Chen, X. Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. expression. Plant Cell ,201224 (6), 2635-2648. DOI: https://doi.org/10.1105/tpc.112.098749 From NLM.
[23]. Kessler, D.; Gase, K.; Baldwin, I. T. Field experiments with transformed plants reveal the sense of floral scents. Science ,2008321 (5893), 1200-1202. DOI. https://doi.org/10.1126/science.1160072 From NLM.
[24]. Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol ,201364 , 665-700. DOI: https ://doi.org/10.1146/annurev-arplant-050312-120116 From NLM.
[25]. Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci U S A ,199895 (8),. 4126-4133. DOI: https://doi.org/10.1073/pnas.95.8.4126 From NLM.
[26]. Lupien, S.; Karp, F.; Wildung, M.; Croteau, R. Regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha) species: cDNA isolation, characterization, and functional expression of (-)-4S-limonene-3-hydroxylase and (-)-4S-limonene-6-hydroxylase. Arch Biochem Biophysics , () characterization, and functional expression of (-)-4S-limonene-3-hydroxylase and (-)-4S-limonene-6-hydroxylase. Arch Biochem Biophys ,1999368 (1), 181-192. DOI: https://doi.org/10.1006/abbi.1999.1298 From NL
[27]. Bao, F.; Zhang, T.; Ding, A.; Ding, A.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Metabolic, Enzymatic Activity, and Transcriptomic Analysis Reveals the Mechanism Underlying the Lack of Characteristic Floral Scent in Apricot Mei Varieties. Front Plant Sci ,202011 , 574982. DOI: https://doi.org/10.3389/ fpls.2020.574982 From NLM
[28]. https://doi.org/10.1093/hr/uhae189From NLM.[29]Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends in Plant Science ,202227 (4), 379-390. DOI: https. //doi.org/10.1016/j.tplants.2021.10.009 .[30]Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the Reaction Catalyzed by 3-Hydroxy-3Methylglutaryl Coenzyme A Reductase a Rate- Limiting Step for Isoprenoid Biosynthesis in Plants? Plant Physiol ,1995109 (4), 1337-1343. DOI:
[29]. Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends in Plant Science ,202227 (4), 379-390. DOI: https. //doi.org/10.1016/j.tplants.2021.10.009 .
[30]. Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the Reaction Catalyzed by 3-Hydroxy-3 Methylglutaryl Coenzyme A Reductase a Rate- Limiting Step for Isoprenoid Biosynthesis in Plants? Plant Physiol ,1995109 (4), 1337-1343. DOI: https://doi.org/10.1104/pp.109.4.1337 From NLM.
[31]. Basak, A.; Chakrabarty, K.; Ghosh, A.; Das, G. K. DFT study on the mechanism of 1,3-hydrogen disposition in Isopentenyl pyrophosphate catalyzed by Isopentenyl pyrophosphate: Dimethylallyl pyrophosphate isomerase. Journal of Theoretical and Computational Chemistry ,201615 (03), 1650025. DOI: https. //doi.org/10.1142/s0219633616500255 .
[32]. Lichtenthaler, H. K.; Schwender, J.; Disch, A.; Rohmer, M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate- independent pathway. FEBS Lett ,1997400 (3), 271-274. DOI: https://doi.org/10.1016/S0014-5793(96)01404-4 From NLM.
[33]. Hu, Y.; Zheng, T.; Dong, J.; Li, W.; Ma, X.; Li, J.; Fang, Y.; Chen, K.; Zhang, K. Regulation of the main terpenoids biosynthesis and accumulation in Horticultural Plant Journal2024 . DOI: https://doi.org/10.1016/j.hpj.2024.08.002。
[34]. Aharoni, A.; Jongsma, M. A.; Kim, T.-Y.; Ri, M.-B.; Giri, A. P.; Verstappen, F. W. A.; Schwab, W.; Bouwmeester, H. J. Metabolic Engineering of Terpenoid Biosynthesis in Plants. Phytochemistry Reviews ,20065 (1), 49-58. DOI: https://doi.org/10.1007/s11101-005-3747-3 .
[35]. Boatright, J.; Negre, F.; Chen, X.; Kish, C. M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in Vivo Benzenoid Metabolism in Petunia Petal Tissue. Plant Physiology ,2004135 (4), 1993-2011. DOI: https://doi.org/10.1104/pp.104.045468 (accessed 4/5/2025).
[36]. Chen, R.; Yang, S.; Zhang, L.; Zhou, Y. J. Advanced Strategies for Production of Natural Products in Yeast. iScience ,202023 (3), 100879. DOI: https://doi. org/10.1016/j.isci.2020.100879 .
[37]. Kaminaga, Y.; Schnepp, J.; Peel, G.; Kish, C. M.; Ben-Nissan, G.; Weiss, D.; Orlova, I.; Lavie, O.; Rhodes, D.; Wood, K.; et al. Plant Phenylacetaldehyde Synthase Is a Bifunctional Homotetrameric Enzyme That Catalyzes Phenylalanine Decarboxylation and Oxidation*. Journal of Biological Chemistry ,2006281 (33), 23357-23366. DOI: https://doi.org/10.1074/jbc.M602708200 .
[38]. Wang, X.; Song, Z.; Ti, Y.; Liu, Y.; Li, Q. Physiological response and transcriptome analysis of Prunus mume to early salt stress. Journal of Plant Journal of Plant Biochemistry and Biotechnology ,202231 (2), 330-342. DOI: https://doi.org/10.1007/s13562-021-00680-2 .
[39]. Zhang, T.; Huo, T.; Ding, A.; Hao, R.; Wang, J.; Cheng, T.; Bao, F.; Zhang, Q. Genome-wide identification, characterization, expression and enzyme activity analysis of coniferyl alcohol acetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS One , (10), e022397 activity analysis of coniferyl alcoholacetyltransferase genes involved in eugenol biosynthesis in Prunus mume. PLoS One ,201914 (10), e0223974. DOI: https. //doi.org/10.1371/journal.pone.0223974 From NLM.
[40]. Chen, Qiu-Chong; Li, Shang-Pu; Miao, Er-Yu; Zhou, Bing-Qian; Wang, X.; Meng, Xiang-Yu; Wang, Xiao-Long; Xu, Kun. HDACi and RS-1 improve the efficiency of CRISPR/Cas12i-mediated HDR editing. Journal of Agricultural Biotechnology ,202432 (10), 2306-2323. from Cnki.
[41]. Ma, X.; Shedlock, C. J.; Medina, T.; Ribas, R. A.; Clarke, H. A.; Hawkinson, T. R.; Dande, P. K.; Wu, L.; Burke, S. N.; Merritt, M. E.; et al. MetaVision3D : Automated Framework for the Generation of Spatial Metabolome Atlas in 3D. bioRxiv2023 . DOI: https://doi.org/10.1101/2023.11.27.568931 From NLM.
[42]. Bao, F.; Ding, A.; Zhang, T.; Luo, L.; Wang, J.; Cheng, T.; Zhang, Q. Expansion of PmBEAT genes in the Prunus mume genome induces characteristic floral scent production. Hortic Res ,20196 , 24. DOI: https://doi.org/10.1038/s41438-018-0104-4 From NLM.
[43]. Xiujun, W.; Zhenqi, S.; Yujing, T.; Kaifeng, M.; Qingwei, L. Comparative transcriptome analysis linked to key volatiles reveals molecular mechanisms of aroma compound biosynthesis in Prunus mume. BMC Plant Biol ,202222 (1), 395. DOI: https://doi.org/10.1186/s12870-022-03779-3 From NLM.
[44]. Hao, R. J.; Qiu, C.; Geng, X. Y.; Jia, H. T.; Zhang, Y. J.; Chang, J.; Feng, X. X.. Functional analysis of Plum Pm ABCG9 in benzyl alcohol volatilization. Chinese Agricultural Science ,202356 (13), 2574-2585. From Cnki.
[45]. Hao, R.; Yang, S.; Zhang, Z.; Zhang, Y.; Chang, J.; Qiu, C. Identification and specific expression patterns in flower organs of ABCG genes related to floral scent from Prunus mume. Scientia Horticulturae ,2021288 , 110218. DOI: https://doi.org/10.1016/j.scienta.2021.110218









