1. Introduction
Skeletal muscles are the most common muscle tissue in our body that controls all of our voluntary actions and takes up 35-45% of our body mass. Skeletal muscles are connected to our tendons, which are all striated with multiple nuclei, and composed of dissimilar components such as muscle fibre, muscle satellite cells and nerves [1]. Briefly, skeletal muscle is a highly organized tissue that is composed of multiple bundles of myofibers. Each myofiber is made from several myofibrils, representing the basic unit of the cell called the sarcomere [2]. Unlike cardiac muscle, whose walls are striated, skeletal muscles are unable to perform spontaneous action potentials because they are composed with no ion channels that are responsible for membrane depolarization. Therefore, the movement of these muscles is only composed of nerve impulses [3]. In normal daily circumstances, these skeletal muscles remain stable and undamaged. However, after injuries or exercise, this muscle tissue will experience atrophy, hypertrophy and even myofiber death. In addition, the fusion of cells conducted by skeletal muscle fusogenes is essential for the development and future action of stable multinucleated myofibers. It is common knowledge that in order to improve muscle capability after exercise after injury is necessary to increase muscle mass. This is because a larger amount of muscle mass contains a large number of muscle nuclei, which are crucial for muscle regeneration. Muscle nuclei play the role that activates post-injury muscle cell proliferation and differentiation to form myoblasts to repair or replace damaged muscle fibre [4].
The insulin system in our body is composed of two similar growth factors, IGF-I and IGF-II. Hence, there are three insulin receptor signals, insulin receptor A (IR-A), insulin receptor B (IR-B), and insulin growth factor IR (IGF-IR). In humans, IGF-I and IGF-II both play the role of tissue growth and development, where insulin controls human body metabolism. Even though IGF and insulin are structurally similar, it still has low affinity towards IGF receptors, where the binding of IGF and its receptor often results in cell proliferation and differentiation [5]. As the role of IGF-II is certain in human muscular myogenesis during embryonic time, this paper will be mainly focused on the effect of IGF-I in adult age. It is important to know that IGF-I only stimulates muscle regrowth if specific mRNA in transcription is switched on in protein synthesis, which allows it to perform a role in myogenesis [6]. It enhances myoblast fusion with damaged tissue, which helps in muscle recovery [7]. IGF-I also activates the PI3K/Akt/mTOR pathway, increasing protein synthesis, generating more polypeptides, and reducing muscle degradation. In muscle recovery, IGF is an important component that stimulates muscle nuclei activation and myoblast differentiation [4].
Resistance exercise is any type of active exercise that involves dynamic or strategic muscle contraction that is resisted by an exterior force. Resistance exercise, also known as resistance training, is a widely known that strengthens aerobic performance and increases our physical capacity. Hence, it improves our motor unit recruitment ratio, and reduces the risk of injury to a certain degree [8]. In addition, mechanical loading also promotes muscle repair by stimulating the muscle nuclei activity and reduces fibrosis [4].
2. The role of resistance exercise in skeletal muscle recovery
2.1. The effect of 12 week resistance exercise on male IGF-I concentration on different age groups.
The reduction of muscle mass and weakness on different age groups is often referred to as sarcopenia. This disease often starts with changes in muscle and nerves, like the loss of muscle mass, mainly in Type II muscle fibers, causing a decrease in body mass, an increase in fat content, a loss in coordination, elevated protein degradation and minor satellite cell activity. In addition, the role of IGF-I is crucial in muscle development. A twelve-week resistance exercise was done by Barzegari and his team to determine the IGF-I concentration in human’s body. An electrical impedance analysis was measured by the Olympia 3.3 Jawon device from Korea. To conduct the experiment, 30 individuals were chosen and separated into two groups: experimental (15 men) and non-experimental (15 men). Participants are put into an ascending 1-RM training within the twelve weeks. Blood serum is collected from both groups of men; the first blood sample is collected 24 hours before the individuals started their resistance exercise. The group of blood samples was collected 24 hours after the last training secession [9].
|
Variable |
Groups |
Pretest |
Post test |
t |
Within-group p-value (t-test0 |
F |
Between group p-value |
Effective size |
|
IGF (ng/ml) |
Training control |
126.9+17.4 128.3+21.7 |
133.6+14.40 127.3+19.7 |
-5.11 7.23 |
0.001 0.057 |
1.4 |
0.004 |
0.65 |
|
Follistatin(ng/ml) |
Training control |
11.75+1.8 12.31+3.13 |
15.88+2.6 12.12+2.8 |
-3.89 -2.10 |
0.004 0.067 |
3.1 |
0.001 |
0.26 |
|
Myostatin(ng/ml) |
Training control |
19.59+5.06 19.28+6.5 |
15.92+4.11 19.63+5.78 |
3.93 -7.11 |
0.001 0.075 |
3.03 |
0001 |
0.38 |
The main goal of this investigation is to determine the effect of resistance exercise by testing the serum levels of IGF-I concentration collected within sedentary elderly men. As IGF-I has been proven to control relative muscle size and plays a significant role in regulating muscle function. It is thought to be beneficial for multiple outcomes of physical activities [10]. The findings of the research (Table 1) are that a long duration of resistance exercise significantly increases the IGF-I level in individuals (p=0.004). Unlike previous studies, Barzegari and his team have significantly lowered the initial IGF-I concentration, including adequate dietary control and changes in plasma volume [9].
In summary, the role of resistance exercise significantly increases the IGF-I concentration in our blood as it increases it by 17%. In the elderly, increased IGF-I and FLST with decreased MSTN creates a favorable condition for muscle maintenance, giving evidence on how IGF-I benefits athletes after skeletal muscle injury as it increases their muscle mass. This research suggests that resistance exercise can be a non-pharmacological way that reduces muscle loss and enhances muscle recovery, and how it outperforms simple resting after muscle injury, as it shows a great difference between them.
2.2. Hormone change to resistance training in females
The investigation of Shiva Aram and his team compared different hormones (GH, IGF-I), changes in response to resistance training in female children with a duration of 8 weeks. The experiment was proceeded with 36 female children, who had a regular exercise routine and no medical intake history in the past year. The participants were separated into three groups: low-intensity suspension training, high-intensity suspension training, and a control group [11].
The data is collected by taking blood samples 48 hours before the first training session and 48 hours after the last training session. The IGF-I hormone was analyzed by enzyme-linked immunosorbent assay. The blood serum is kept under -80 degrees Celsius until analysis and is centrifuged for 10 min at 3000 rpm with the blood samples [11].
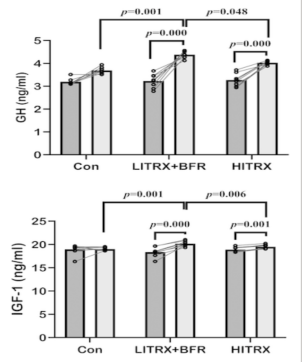
The results shown in Figure 1 indicate that the IGF-I hormone experiences a significant change (p=0.007). In the diagram, HITRX stands for high-intensity resistance exercise, LITRX is low-intensity resistance exercise and CON is the controlled group. However, looking at data, people performing low-intensity resistance exercise with blood flow restriction (BFR) have a much higher IGF-I serum concentration compared to those under high-intensity resistance exercise. This result is indeed supported by previous studies as the result of Abe had proven that BFR can significantly increase IGF-I concentration [12]. On the other hand, there are researches that show that BFR might not significantly increase the IGF-I concentration in blood serum [11]. The different results found within these investigations can be found due to the different ways of wrapping, use of different pressure during exercise, different weight training and duration of exercise [11]. While in this investigation, the elevated IGF-I concentration in blood serum can be an effect of the elevated concentration of GH [12].
In summary, this study was the first to investigate the IGF-I concentration difference between high-intensity training and blood flow restriction flow-intensity training. Therefore, if injuries are considered and the priority of exercise is to enhance muscular recovery, low intensity training with blood flow restriction is more beneficial as both past and present studies suggest that both GH and IGF-I concentrations show a great difference with people at rest.
2.3. Hormonal response of resistance training between prepubertal vs. pubertal male children
As most investigation done on resistance training that determines IGF-I concentration changes is done on adults. Previous studies suggest that modest changes occur after resistance exercise [13]. Therefore, the study of Jansson and his team investigated hormonal and cytokine changes in prepubertal and pubertal male children proceeding with free weight resistance exercise. The result of this investigation might show the role of maturation in IGF-I concentration, however, also giving evidence for the role of IGF-I generation during resistance exercise.
During the investigation, a total of 48 participants (23 prepubertal and 25 pubertal) were separated into two groups based on their biological maturity range (prepubertal (Tanner level I–II) and pubertal (Tanner level III–V)) [13]. All of the participants were asked to proceed with 10 RM bench and leg presses. IGF-I in the investigation is assessed by a high-sensitivity bead-based multiplex assay using the Luminex technology (MILLIPLEX map kit, Millipore) [13]. The serum hormone is tested before exercise, and immediately, 15 and 30 minutes after the resistance exercise.
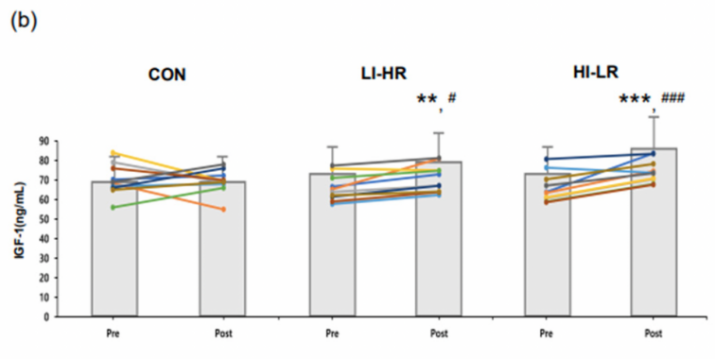
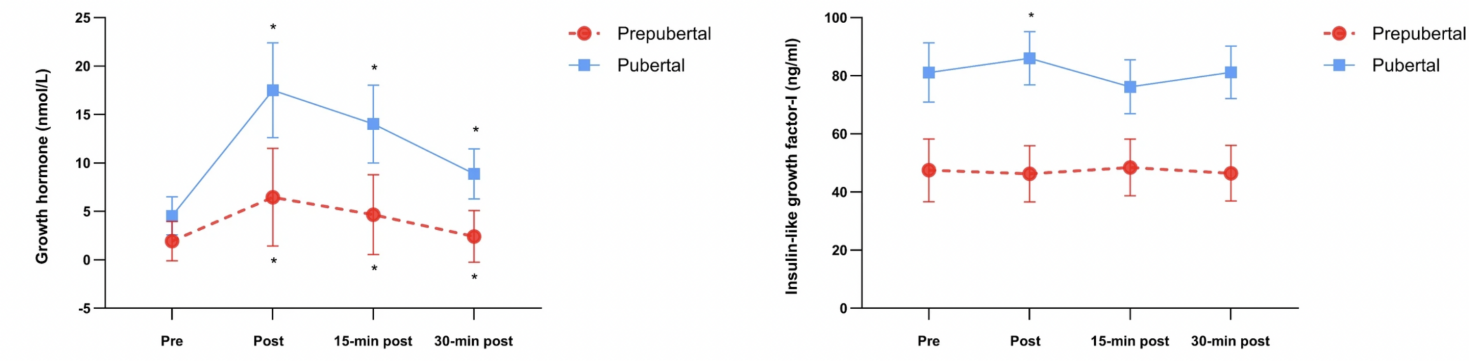
The IGF-I concentration over all time shows a high consistent value over the pubertal group compared to the prepubertal group [13]. However, as shown in Figure 2, after resistance exercise, there was an obvious increase in IGF-I concentration in the pubertal group. To compare, pubertal groups show a higher IGF-I difference (p=0.049) [13] with an adequate increase in post-exercise condition. In contrast, in prepubertal children, the data shows no significant difference [13] even though the GH shows a great elevation in both groups. Nevertheless, Jansson and his team also suggest several limitations that might change their final result. To explain, prepubertal groups have a higher inflammatory response in post-exercise conditions because there is an elevated IL-6 concentration after resistance exercise, which may suppress IGF-I expression [13].
In summary, the research of Jansson and his team shot similar results: resistance exercise stimulated the synthesis of IGF-I in the human body. However, the result also shows that mature children are more capable of generating an enlarged hormonal response in post-exercise conditions. Even though the IGF response in youths remains unclear, this research shows direct evidence that resistance exercise is a pathway that enlarges IGF secretion for people in and after puberty, suggesting its capability in muscular recovery.
2.4. Effect of low intensity and high intensity elastic band resistance on myokine level
Lee and his team study how 12 weeks of low-intensity, high repetition (LI-HR) versus high-intensity, low-reception (HI-LR) resistance affect myokine levels in older adults (over 65 years old). During the investigation, 36 participants were randomly distributed into three equal groups, with one as the control. All participants selected an elastic band according to their relative strength and started the research by training their grip width. High-intensity major targeted load, where low-intensity target quantity is 50%, more repletion than those in high intensity. Within the experiment, all participants were restricted from alcohol, caffeine, and excess exercise [14]. In order to determine the myokine level, a blood sample is collected from the forearm vein 10 hours after exercise. All the blood samples are immediately centrifuged and stored under -80 degrees Celsius, waiting for analysis. In addition, IGF-I samples were treated to avoid binding to a specific receptor [14].
The results show that both types of resistance exercise increase IGF-I concentration in older adults (Figure 3). Both groups show that IGF-I concentration in post-exercise condition is greater than pre-exercise condition, with HI-LR showing a great significance (LI-HR p=0.003, HI-LR p greater than 0.001) [14]. However, there are still limitations within this research because the IGF-I is pretreated in the investigation, hence affecting the total accuracy of IGF-I concentration. Further research should determine the effect of IGF-I concentration under resistance exercise along with nutrition intake [14]. Therefore, it is proven that LI-HR can increase human potential to upgrade their physical fitness, muscle strength and power, possibly giving evidence to be beneficial for muscle regrowth.
In a word, participants from different age groups are analyzed in this review paper, and all the results have shown that the changes in IGF-I concentration are not relevant to age groups. Furthermore, resistance exercise is proven to be a non-medical way to increase IGF-I concentration in the human body, affecting the rate of muscle protein synthesis and stem cell differentiation.
3. Conclusion
In summary, the review synthesis evidence from petiole stage suggesting that resistance exercise significantly enhances IGF-I concentration in human across different age groups and gender. It is a common knowledge that IGF-I stimulates myoblast fusion with damaged muscle tissue and activate satellite cells and muscle nuclei, thereby increasing muscle mass. It increases protein synthesis, and reduces muscle nuclei degradation. Therefore, this review gives evidence for how resistance exercise benefits muscular recovery by increasing IGF-I concentration. It is a great finding that resistance exercise can be a non-pharmacological pathway to enhance muscular recovery, which could reduce the overall problem of drug resistance. Resistance exercise, performs passive recovery by creating a favorable anabolic environment which elevates IGF-I and reduces myostatin. However, the researches done recently collects stat from participants blood serum, however IGF-I also performs other function in human body such as regulating bone health and mimics insulin by controlling glucose uptake. Therefore, the IGF-I collected from the participants might not be fully used in muscular repair, affecting its overall reliability. Further researches should investigate IGF-I isoforms which only contributes in muscular repair. In addition, instigation should be proceeded to compare different antirational intervention with resistance exercise and determine its effect on IGF-I isoform concentration. Most importantly, researchers can investigate and develop IGF-I targeted therapies for muscle wasting disease. Finally, resistance exercise is a safe and effective strategy to enhance muscular recovery and IGF-I upregulation. Future work should refine protocols and focus on molecular insights to maximize its potential in physical therapies.
References
[1]. Lian, Di, Ming-Ming Chen, Hanyu Wu, Shoulong Deng, and Xiaoxiang Hu. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 11, no. 4, April 11, 2022: 755. https://doi. org/10. 3390/antiox11040755.
[2]. Mukund, Kavitha, and Shankar Subramaniam. Skeletal Muscle: A Review of Molecular Structure and Function, in Health and Disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 12, no. 1, August 13, 2020. https://doi. org/10. 1002/wsbm. 1462.
[3]. Hopkins, Philip M. Skeletal Muscle Physiology. Continuing Education in Anaesthesia Critical Care & Pain 6, no. 1, February 2006: 1–6. https://doi. org/10. 1093/bjaceaccp/mki062.
[4]. Laumonier, Thomas, and Jacques Menetrey. Muscle Injuries and Strategies for Improving Their Repair. Journal of Experimental Orthopaedics 3, no. 1, July 22, 2016. https://doi. org/10. 1186/s40634-016-0051-7.
[5]. Joseph. The Impact of Westernization on the Insulin/IGF-I Signaling Pathway and the Metabolic Syndrome: It Is Time for Change. International Journal of Molecular Sciences 24, no. 5, February 25, 2023: 4551–51. https://doi. org/10. 3390/ijms24054551.
[6]. Nicholas A. M. Pansters, Ramon, Emiel F. M. Wouters, and Annemie M. W. J. Schols. Synergistic Stimulation of Myogenesis by Glucocorticoid and IGF-I Signaling. Journal of Applied Physiolog 114, no. 9, May 1, 2013: 1329–39. https://doi. org/10. 1152/japplphysiol. 00503. 2012.
[7]. CHARGÉ, SOPHIE B. P. , and Michael A. Rudnicki. Cellular and Molecular Regulation of Muscle Regeneration. Physiological Reviews 84, no. 1, January 2004: 209–38. https://doi. org/10. 1152/physrev. 00019. 2003.
[8]. Khachane, Aditya. Resistance Training. Physiopedia, 2020. https://www. physio-pedia. com/Resistance_Training?utm_source=physiopedia&utm_medium=related_articles&utm_campaign=ongoing_internal.
[9]. Barzegari Marvast, H, A Akbarnejad, and J Norouzi. Effect of 12 Weeks Incremental Resistance Training on Serum Levels of Myostatin, Follistatin, and IGF-I in Sedentary Elderly Men. Iranian Journal of War and Public Health 16, no. 1, January 10, 2024: 1–8. https://doi. org/10. 58209/ijwph. 16. 1. 1.
[10]. Yoshida, Tadashi, and Patrice Delafontaine. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 9, no. 9, August 26, 2020: 1970. https://doi. org/10. 3390/cells9091970.
[11]. Aram, Shiva, Kazem Khodaei, and Mohamadreza Zolfaghar Didani. The Effect of Low‐Intensity Suspension Training with Blood Flow Restriction on GH, IGF‐1, and Their Association with Physical Fitness in Young Women. Physiological Reports 12, no. 15, August 2024. https://doi. org/10. 14814/phy2. 16154.
[12]. Abe, T. , T. Yasuda, T. Midorikawa, Y. Sato, C. F. Kearns, K. Inoue, K. Koizumi, and N. Ishii. Skeletal Muscle Size and Circulating IGF-1 Are Increased after Two Weeks of Twice Daily ‘KAATSU’ Resistance Training. International Journal of KAATSU Training Research 1, no. 1 (2005): 6–12. https://doi. org/10. 3806/ijktr. 1. 6.
[13]. Jansson, Daniel, Elena Lundberg, Anna-Clara Rullander, Magnus Domellöf, Ann-Sofie Lindberg, Helena Andersson, and Apostolos Theos. Hormonal and Inflammatory Responses in Prepubertal vs. Pubertal Male Children Following an Acute Free-Weight Resistance Training Session. European Journal of Applied Physiology, September 11, 2024. https://doi. org/10. 1007/s00421-024-05603-2.
[14]. Lee, Moon Jin, Jun-Young Sung, and Jiyoun Kim. Effect of Low-Intensity High-Repetition versus High-Intensity Low-Repetition Elastic Band Resistance Training on Functional Physical Fitness and Myokine Levels in Older Adults. Applied Sciences 15, no. 2, January 14, 2025: 757. https://doi. org/10. 3390/app15020757.
Cite this article
Zhao,S. (2025). The Role of Resistance Exercise in Skeletal Muscle Recovery, and Changes in Igf-1 Secretion. Theoretical and Natural Science,117,32-38.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lian, Di, Ming-Ming Chen, Hanyu Wu, Shoulong Deng, and Xiaoxiang Hu. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 11, no. 4, April 11, 2022: 755. https://doi. org/10. 3390/antiox11040755.
[2]. Mukund, Kavitha, and Shankar Subramaniam. Skeletal Muscle: A Review of Molecular Structure and Function, in Health and Disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 12, no. 1, August 13, 2020. https://doi. org/10. 1002/wsbm. 1462.
[3]. Hopkins, Philip M. Skeletal Muscle Physiology. Continuing Education in Anaesthesia Critical Care & Pain 6, no. 1, February 2006: 1–6. https://doi. org/10. 1093/bjaceaccp/mki062.
[4]. Laumonier, Thomas, and Jacques Menetrey. Muscle Injuries and Strategies for Improving Their Repair. Journal of Experimental Orthopaedics 3, no. 1, July 22, 2016. https://doi. org/10. 1186/s40634-016-0051-7.
[5]. Joseph. The Impact of Westernization on the Insulin/IGF-I Signaling Pathway and the Metabolic Syndrome: It Is Time for Change. International Journal of Molecular Sciences 24, no. 5, February 25, 2023: 4551–51. https://doi. org/10. 3390/ijms24054551.
[6]. Nicholas A. M. Pansters, Ramon, Emiel F. M. Wouters, and Annemie M. W. J. Schols. Synergistic Stimulation of Myogenesis by Glucocorticoid and IGF-I Signaling. Journal of Applied Physiolog 114, no. 9, May 1, 2013: 1329–39. https://doi. org/10. 1152/japplphysiol. 00503. 2012.
[7]. CHARGÉ, SOPHIE B. P. , and Michael A. Rudnicki. Cellular and Molecular Regulation of Muscle Regeneration. Physiological Reviews 84, no. 1, January 2004: 209–38. https://doi. org/10. 1152/physrev. 00019. 2003.
[8]. Khachane, Aditya. Resistance Training. Physiopedia, 2020. https://www. physio-pedia. com/Resistance_Training?utm_source=physiopedia&utm_medium=related_articles&utm_campaign=ongoing_internal.
[9]. Barzegari Marvast, H, A Akbarnejad, and J Norouzi. Effect of 12 Weeks Incremental Resistance Training on Serum Levels of Myostatin, Follistatin, and IGF-I in Sedentary Elderly Men. Iranian Journal of War and Public Health 16, no. 1, January 10, 2024: 1–8. https://doi. org/10. 58209/ijwph. 16. 1. 1.
[10]. Yoshida, Tadashi, and Patrice Delafontaine. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 9, no. 9, August 26, 2020: 1970. https://doi. org/10. 3390/cells9091970.
[11]. Aram, Shiva, Kazem Khodaei, and Mohamadreza Zolfaghar Didani. The Effect of Low‐Intensity Suspension Training with Blood Flow Restriction on GH, IGF‐1, and Their Association with Physical Fitness in Young Women. Physiological Reports 12, no. 15, August 2024. https://doi. org/10. 14814/phy2. 16154.
[12]. Abe, T. , T. Yasuda, T. Midorikawa, Y. Sato, C. F. Kearns, K. Inoue, K. Koizumi, and N. Ishii. Skeletal Muscle Size and Circulating IGF-1 Are Increased after Two Weeks of Twice Daily ‘KAATSU’ Resistance Training. International Journal of KAATSU Training Research 1, no. 1 (2005): 6–12. https://doi. org/10. 3806/ijktr. 1. 6.
[13]. Jansson, Daniel, Elena Lundberg, Anna-Clara Rullander, Magnus Domellöf, Ann-Sofie Lindberg, Helena Andersson, and Apostolos Theos. Hormonal and Inflammatory Responses in Prepubertal vs. Pubertal Male Children Following an Acute Free-Weight Resistance Training Session. European Journal of Applied Physiology, September 11, 2024. https://doi. org/10. 1007/s00421-024-05603-2.
[14]. Lee, Moon Jin, Jun-Young Sung, and Jiyoun Kim. Effect of Low-Intensity High-Repetition versus High-Intensity Low-Repetition Elastic Band Resistance Training on Functional Physical Fitness and Myokine Levels in Older Adults. Applied Sciences 15, no. 2, January 14, 2025: 757. https://doi. org/10. 3390/app15020757.









