1. Introduction
Idiopathic scoliosis (IS) refers to a three-dimensional spinal deformity characterized by lateral curvature in one or more spinal segments accompanied by vertebral rotation [1] . It often leads to varying degrees of lower back pain and insomnia, and is associated with negative psychological symptoms such as depression and low self-esteem. In severe cases, it may result in impaired cardiopulmonary function, severely affecting the physical and mental health as well as the growth and development of children and adolescents [2,3] . The etiology of IS remains unknown, and it is considered a disease potentially influenced by multiple susceptibility factors, including genetics, environment, hormones, metabolism, and neurology [1] . Studies have shown a strong correlation between adolescent idiopathic scoliosis (AIS) and congenital genetic factors [4] , indicating that individuals carrying certain genes are more likely to develop scoliosis than the general population. In addition, Dou et al. [5] pointed out that acquired environmental factors, such as daily lifestyle habits, are also indispensable susceptibility factors for IS. Exploring both congenital and acquired factors, and summarizing the possible congenital genetic and acquired environmental contributors alongside their interactions, is of great importance for the early detection, prevention, and intervention of IS in children and adolescents.
2. Congenital factors influencing idiopathic scoliosis in children and adolescents
Research by Watanabe et al. [6] indicates that adolescents whose mothers have scoliosis are 1.5 times more likely to develop AIS compared to those with no family history of the condition. Congenital genetic factors play a crucial role among the susceptibility elements for IS, primarily involving genetically related factors such as hormones (e.g., estrogen, leptin, and melatonin levels), age at menarche, and obesity (see Figure 1). These congenital factors are regulated by gene expression and can influence the development of scoliosis either directly or indirectly by interacting with other congenital or acquired factors. Investigating the congenital factors associated with IS is essential for the early identification of potential risk elements, enabling timely and effective preventive interventions.
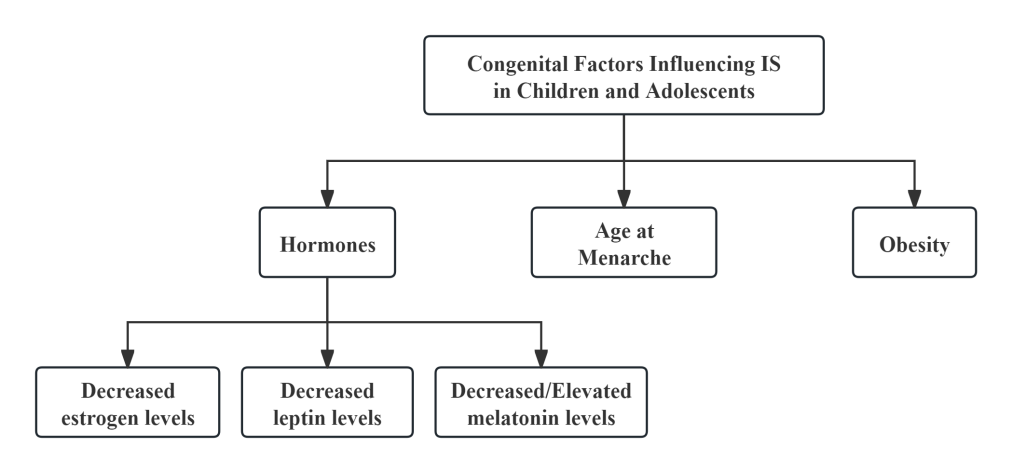
2.1. Hormones
2.1.1. Estrogen
The prevalence of IS in female children and adolescents is significantly higher than in males, suggesting a potential association between IS and estrogen. A meta-analysis [7] has shown that polymorphisms in estrogen receptor α (ERα) and estrogen receptor β (ERβ) genes may be associated with AIS susceptibility, with differences observed across ethnic groups. However, the mechanisms by which estrogen influences the onset and progression of IS remain controversial. Two primary hypotheses have been proposed [8] : first, decreased estrogen levels may delay the onset of menarche in females, indirectly reducing bone metabolism and increasing the risk of spinal deformities; second, decreased estrogen levels may directly impair bone metabolism and remodeling, thereby increasing the prevalence of IS.
Estrogen receptors also appear to influence other susceptibility factors for scoliosis. In a study involving blood samples from 49 participants, Ahmadi et al. [9] found a positive correlation between estrogen and leptin, with ER also affecting obesity-related metabolism and increasing resting energy expenditure through leptin. Moreover, decreased estrogen levels can lead to reduced muscle mass and protein synthesis rates, as well as increased visceral fat. Another study [10] suggested a link between estrogen and the paraspinal muscles. In an investigation of 35 adolescent females, it was found that ERα expression in deep paraspinal muscles was significantly higher than in superficial layers, and that ERα and ERβ expression was asymmetrical between the left and right sides of the deep muscle, potentially contributing to spinal deformity.
2.1.2. Leptin
Leptin is a peptide hormone primarily secreted by adipose tissue. Liu et al. [11] recruited 570 IS patients of Han Chinese descent aged 10–18 and an equal number of age-matched healthy controls to conduct genotyping and compare average Cobb angles and body mass index(BMI) among different genotypes. The study found that the SNP rs2767485 in the leptin receptor gene was associated with IS susceptibility in the Han Chinese population. Additionally, a prospective cohort study [12] discovered a negative correlation between leptin levels at age 10 and the severity of scoliosis at age 15, while adiponectin levels at age 10 were positively associated with scoliosis severity.
Leptin is also associated with the age of menarche. Insufficient leptin levels can delay menarche [13] , thereby reducing bone mineral density [14] and increasing the risk of scoliosis. One study [15] confirmed this and further suggested that age at menarche may be associated with the G-2548 A polymorphism in the leptin gene. Moreover, leptin can influence body composition by promoting lipolysis, enhancing protein synthesis, and increasing muscle mass.
2.1.3. Melatonin
Melatonin, a hormone secreted by the pineal gland, plays a role in various physiological functions. Thillard [16] observed that 65% of chickens developed spinal curvature following pinealectomy, suggesting for the first time a potential link between melatonin and scoliosis. Subsequent studies concluded that increased Cobb angles in IS patients are associated with reduced melatonin levels [17] . However, Gargano et al. [18] , through a review of the literature, noted that although melatonin alterations are observed in IS, a direct causal relationship remains controversial.
Melatonin may affect bone mineral density and exhibits concentration-dependent inhibitory effects on osteoblast proliferation, differentiation, and apoptosis. One study investigating the mechanism of melatonin [19] found that it induces mitochondrial apoptosis in osteoblasts by modulating the intercellular adhesion molecule-1 pathway, cytosolic calcium levels, and the extracellular signal-regulated kinase (ERK) pathway. These findings provide a foundation for further clinical research on melatonin-based treatment for IS in children and adolescents. Meanwhile, elevated melatonin levels may lower estrogen levels by inhibiting aromatase activity and regulating the hypothalamic–pituitary–gonadal axis, thereby decreasing bone mineral density and increasing the incidence of scoliosis. Conversely, decreased melatonin levels may affect body composition and BMI, leading to fat accumulation, accelerated protein catabolism, and muscle loss. There may also be a relationship between melatonin and the paraspinal muscles, as one study [20] reported that the mRNA expression level of melatonin receptor 2 was higher on the concave side of the paraspinal muscles than on the convex side.
2.2. Age at menarche
Age at menarche (AAM) is an important marker of female pubertal development and is associated with IS in children and adolescents. Lim et al. [21] studied 38,879 female IS patients and found that those with delayed AAM tended to have larger Cobb angles. Multiple studies have indicated that AAM has a genetic predisposition, with LIN28B being a key regulatory factor in female growth and puberty [22] . On the other hand, the onset of puberty is related to the reactivation of the hypothalamic-pituitary-gonadal (HPG) axis, a process also regulated by genetic factors [23].
To date, whether delayed AAM is associated with genetic polymorphisms remains controversial. Feigelson et al. [24] reported that delayed AAM was associated with polymorphisms in the cytochrome P450c17α gene. However, a subsequent meta-analysis [25] involving over 11,000 individuals found that the MspA1 restriction enzyme site in this gene polymorphism was not an independent risk factor for AAM. Moreover, AAM may indirectly contribute to scoliosis by affecting estrogen exposure and bone mineral density. A delayed AAM shortens the period of estrogen exposure, leading to reduced bone mineral density [14] and thus increasing the risk of IS.
2.3. Obesity
While numerous studies have identified a correlation between IS and low BMI in children and adolescents, the role of high BMI has often been overlooked. However, IS is also associated with obesity. Goodbody et al. [26] included 50 adolescents each from normal weight, overweight, and obese IS patient groups, and measured BMI, curvature severity, and curvature location. They found that overweight and obese patients exhibited significantly greater spinal curvature and were less likely to be detected in early screenings. Another study [1] found that among 196 obese adolescents, the prevalence of IS reached 12.2%, which is twice the rate observed in the general population.
Many studies have shown that 40–70% of obesity cases are genetically related, involving variants in genes such as leptin (LEP), leptin receptor (LEPR), melanocortin 4 receptor (MC4R), pro-opiomelanocortin (POMC), neuropeptide Y (NPY), and fat mass and obesity-associated gene (FTO) [27] . Among these, MC4R deficiency is the most common cause of monogenic obesity. Saeed et al. [28] conducted genetic studies on 73 severely obese Pakistani children from consanguineous families and concluded that genetic variations in LEP, LEPR, and MC4R explained 30% of severe obesity cases in this population.
3. Acquired factors influencing idiopathic scoliosis in children and adolescents
IS in children and adolescents is not only closely related to congenital genetic factors but is also influenced by acquired environmental factors [5] . It is generally believed that a combination of genetic predisposition and acquired conditions represents the most probable cause of IS in this population. Acquired factors such as BMI and body composition, physical activity, bone mineral density and paraspinal muscles are considered susceptibility factors for IS (see Figure 2), and interactions exist among these acquired elements. These factors are closely tied to lifestyle habits, with poor habits increasing the incidence of scoliosis. Moreover, changes in congenital factors may also affect acquired traits, leading to spinal alterations. Therefore, acquired factors are an important component in the susceptibility to IS in children and adolescents.
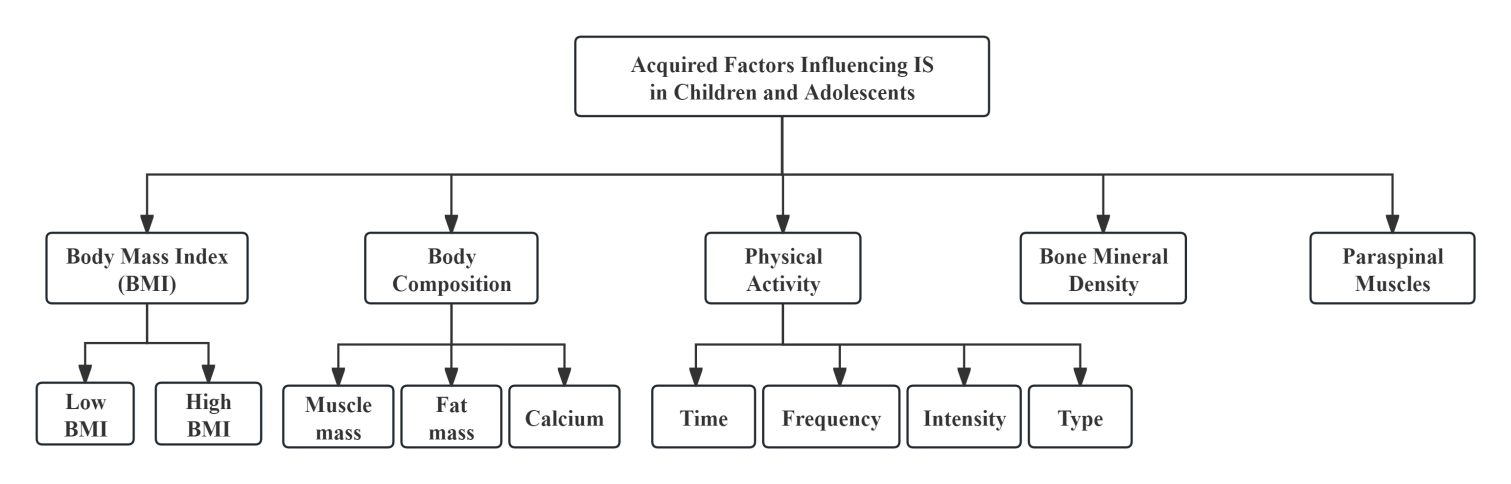
3.1. BMI and body composition
3.1.1. BMI
BMI is an important indicator for assessing body weight status and overall health. Zou et al. [29] , using a scoliometer to measure the Angle of Trunk Rotation (ATR) in 45,547 primary and secondary school students, found that individuals with lower BMI had larger ATR values. However, a cohort study [26] employing the “gold standard” of X-ray to measure spinal curvature via Cobb angle revealed that patients with higher BMI exhibited larger Cobb angles than those with normal BMI. Margalit et al. [30] compared ATR and Cobb angle measurements and concluded that among patients with identical Cobb angles, those with higher BMI had greater ATR values, suggesting that lower ATR values in overweight and obese populations may require attention.
Both low and high BMI may be susceptibility factors for scoliosis. Earlier studies largely overlooked the relationship between high BMI and scoliosis. One reason might be that the larger body mass in high BMI individuals conceals vertebral rotation [31] , making it harder to detect via ATR screening. Consequently, scoliosis identified in overweight and obese individuals often presents with larger Cobb angles. Another issue lies in the limitations of BMI classification itself. Durmala et al. [32] assessed the nutritional status of IS patients using both BMI and bioelectrical impedance analysis (BIA), and found that BMI classification underestimated the number of overweight and obese IS patients. There may be a bias in the use of BMI for nutritional status classification in IS patients.
3.1.2. Body composition
Body composition refers to the relative proportions of fat, bone, and muscle in the human body. Matusik et al. [33] used BIA to measure the body composition of IS patients and found that the severe IS group had a significantly higher BMI than the moderate IS group, with lower predicted muscle mass and higher fat mass. Similarly, Miyagi et al. [34] applied BIA to assess body composition in female AIS patients but found the opposite BMI result: the severe IS group had significantly lower BMI than the moderate group, yet also showed lower muscle mass and higher fat content. Although both studies followed the same research design and measurement methods, they yielded consistent findings regarding body composition but contradictory results for BMI. This discrepancy may stem from BMI being influenced by factors such as ethnicity, gender, and age, suggesting that body composition may be a more fundamental susceptibility factor for IS.
Both studies mentioned above indicated that an increase in fat mass correlates with greater IS severity, but this conclusion remains debated. A prospective study [12] exploring the relationship between body composition at age 10 and IS at age 15 found that increases in lean mass and fat mass were associated with reduced IS risk, potentially related to leptin levels [35] . Wang et al. [36] used dual-energy X-ray absorptiometry to measure body composition in males and found no significant difference in fat mass between AIS patients and healthy controls. In summary, lower muscle mass and reduced lean body mass appear to be risk factors for IS, while the role of fat mass may vary by gender and measurement method and warrants further research.
3.2. Physical activity
3.2.1. Positive effects on IS patients
Glavaš et al. [37] investigated 18,216 primary school students and found that IS patients had less frequent and shorter durations of physical activity. Physical activity is closely related to IS, and engaging in regular and appropriately intense physical activity can help reduce the incidence of IS. Tobias et al. [38] included 4,640 participants who had not developed scoliosis at age 10 and found that engaging in vigorous physical activity at age 10 reduced the incidence of AIS by age 15 by 53%. As for intensity, some studies suggest that moderate-intensity exercise is more effective than low- or high-intensity exercise [39] . Thus, physical activity has a positive impact on IS in children and adolescents, though the relationship between exercise intensity and IS still requires further research.
Physical activity may also reduce the risk of IS by influencing BMI and body composition. Lv [40] found in a 12-week intervention involving 45 primary school students with IS that exercise altered BMI as well as muscle and fat composition. Physical activity increases muscle mass, reduces fat accumulation, and helps normalize BMI. Additionally, it strengthens the paraspinal muscles, thereby enhancing spinal stability. Exercising outdoors can also facilitate the absorption of vitamin D and calcium among adolescents, which positively influences AIS.
3.2.2. Negative effects on IS patients
Asymmetrical physical activities may trigger IS. Several systematic reviews have shown a positive correlation between AIS and sports such as dance, rhythmic gymnastics, swimming, volleyball, and ballet [39,41] . These activities may cause asymmetrical spinal motion, leading to spinal deformation and rotation, thereby increasing the risk of IS. Although swimming activates almost all major muscle groups, whether it benefits spinal health depends on factors such as swimming posture and duration, which warrant further investigation.
Furthermore, weight-bearing activities have a negative effect on IS in children and adolescents. Hell et al. [42] found that carrying a 4 kg backpack destabilizes gait in primary school students, who compensate through the pelvis, trunk, and hips—this increases spinal load and alters spinal curvature. Presta et al. [43] also noted that even minor asymmetric loads can alter gait and pose a risk to spinal health. In addition, asymmetrical and excessive load-bearing physical activities can affect paraspinal muscles, potentially leading to muscle fibrosis, fat infiltration, and reduced fatigue resistance [44] , thereby increasing the risk of IS.
3.3. Bone mineral density
Burner et al. [45] were the first to propose that AIS patients tend to have low bone mineral density (BMD). Li et al. [46] further explored this through a longitudinal study and found that low BMD in AIS patients correlates with the degree of spinal curvature, and may persist into adolescence and even beyond the peak bone mass phase.
BMD is also influenced by BMI, body composition, and physical activity. Korkmaz et al. [47] found that systemic inflammation associated with obesity negatively affects bone metabolism, leading to low bone mass and osteoporosis. A longitudinal cohort study [48] showed that two years of calcium and vitamin D2 supplementation in AIS patients significantly improved BMD and bone quality, but most of the therapeutic effects disappeared four years after stopping supplementation. This indicates that calcium and vitamin D2 play an important role in maintaining bone health in AIS patients. Yuan et al. [49] also pointed out that exercise appears to act directly or indirectly on nearly all types of bone cells, thereby increasing bone mineral density, mass, strength, and mechanical performance.
4. Interaction between congenital and acquired factors
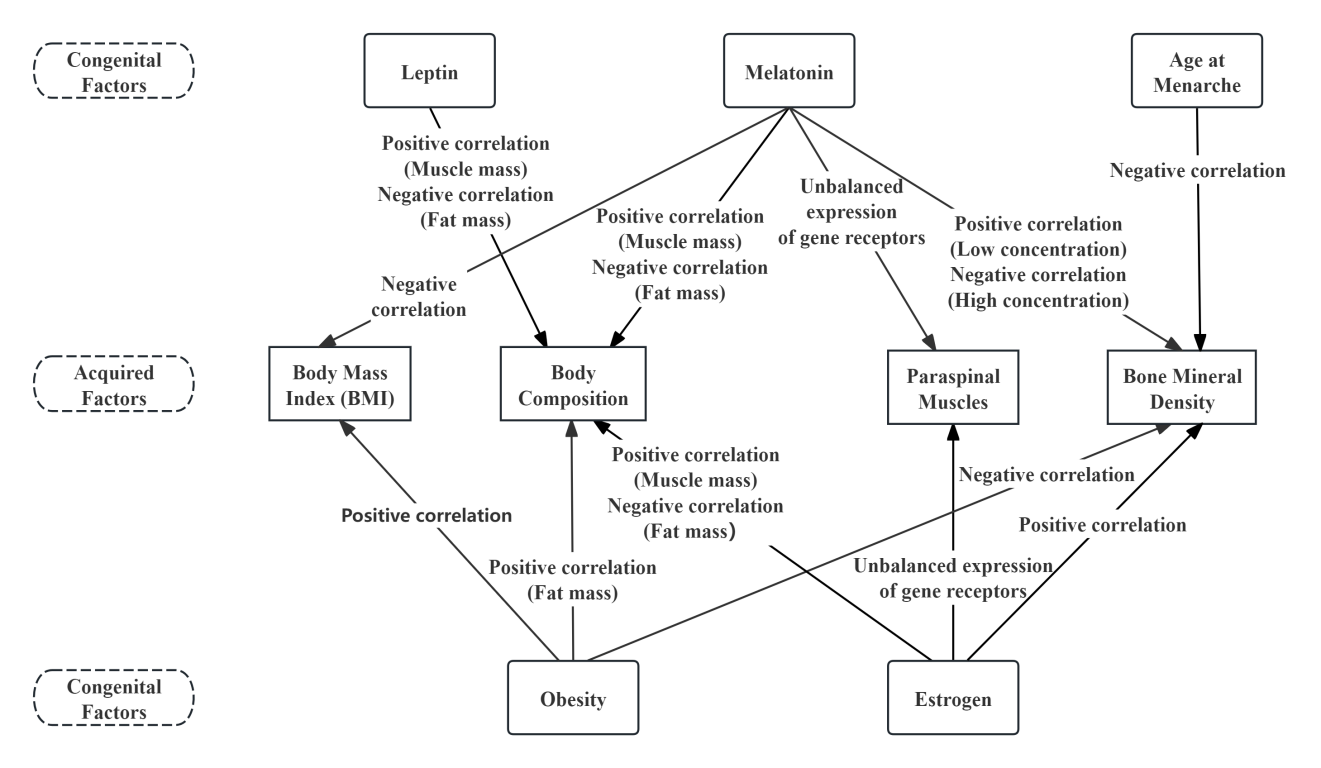
Congenital genetic factors are influenced by heredity and interact with one another, thereby indirectly or directly affecting acquired environmental factors (see Figures 3 and 5). A reduction in leptin secretion can lead to delayed AAM [13] , which in turn shortens the duration of estrogen exposure. This may result in changes in body composition, further altering BMI. In terms of hormones, estrogen, leptin, and melatonin all influence body composition, showing positive correlations with protein content and muscle mass, and negative correlations with fat mass. Abnormal secretion of estrogen and melatonin can also directly affect the asymmetrical development of the paraspinal muscles [10,20] . Regarding the impact of congenital factors on BMD, estrogen can indirectly affect BMD by delaying AAM, while estrogen [8] and melatonin [19] can also exert direct effects on BMD. Additionally, the leptin gene influences obesity [27] , and lack of melatonin increases body weight, thereby indirectly elevating BMI [50] and consequently altering BMD [47].
Acquired environmental factors also interact with one another (see Figure 4). BMI and body composition are influenced not only by congenital factors but also by the frequency, duration, and intensity of physical activity. Furthermore, asymmetrical and excessive weight-bearing activities can affect the paraspinal muscles, altering their properties [44] , whereas appropriate physical activity can strengthen these muscles and enhance spinal stability. As for BMD, although it may be adversely affected by various congenital factors, it can be positively improved through the normalization of BMI [47] , supplementation with adequate calcium and vitamin D2 [48] , and appropriate physical activity [49] , all of which can help restore BMD to healthy levels.
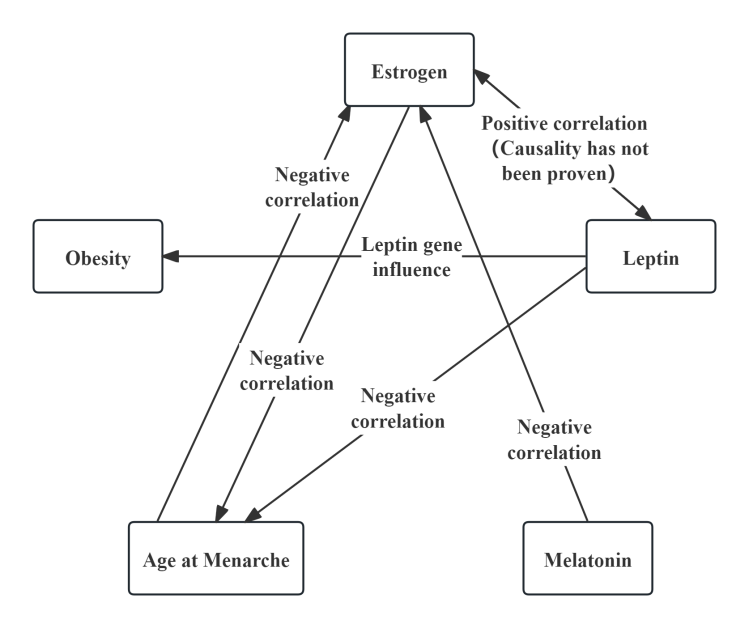
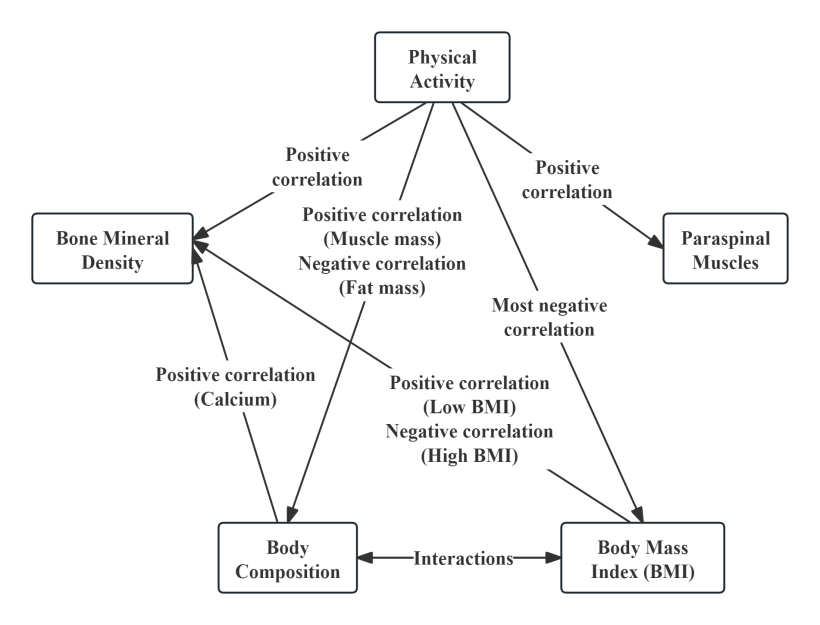
5. Conclusion
In summary, the development of IS in children and adolescents is the combined effect of congenital genetic factors and acquired environmental factors. These factors do not operate in simple linear relationships; rather, they engage in intricate interactions that collectively determine whether the spine develops normally or undergoes abnormal curvature. For instance, body composition is not only directly influenced by estrogen, leptin, melatonin, obesity, BMI, and physical activity, but is also indirectly affected by these same factors, as well as AAM.
Therefore, when exploring the pathogenesis of IS and formulating prevention and treatment strategies, it is essential to adopt an integrated perspective. In-depth analysis should be conducted on the complex interactions between congenital factors and acquired factors. To develop precise and effective interventions aimed at achieving optimal clinical outcomes and promoting full spinal recovery in patients, a thorough understanding of the underlying mechanisms is essential.
References
[1]. Catanzariti, J. -F. , et al. (2023). Idiopathic adolescent scoliosis and obesity: Prevalence study. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 32(6), 2196-2202. https://doi. org/10. 1007/s00586-023-07709-1
[2]. Wong, A. Y. L. , et al. (2019). How common is back pain and what biopsychosocial factors are associated with back pain in patients with adolescent idiopathic scoliosis? Clinical Orthopaedics and Related Research, 477(4), 676-686. https://doi. org/10. 1097/CORR. 0000000000000569
[3]. Mitsiaki, I. , et al. (2022). Adolescent idiopathic scoliosis and mental health disorders: A narrative review of the literature. Children (Basel, Switzerland), 9(5), 597. https://doi. org/10. 3390/children9050597
[4]. Cheng, T. , et al. (2022). Idiopathic scoliosis: A systematic review and meta-analysis of heritability. EFORT Open Reviews, 7(6), 414-421. https://doi. org/10. 1530/EOR-22-0026
[5]. Dou, Q. , et al. (2023). Academic-related factors and daily lifestyle habits associated with adolescent idiopathic scoliosis: A case-control study. Environmental Health and Preventive Medicine, 28, 23. https://doi. org/10. 1265/ehpm. 22-00243
[6]. Watanabe, K. , et al. (2017). Physical activities and lifestyle factors related to adolescent idiopathic scoliosis. The Journal of Bone and Joint Surgery. American Volume, 99(4), 284-294. https://doi. org/10. 2106/JBJS. 16. 00459
[7]. Sobhan, M. R. , et al. (2020). Association of ESRα XbaI A > G, ESRα PvuII T > C and ESRβ AlwNI T > C polymorphisms with the risk of developing adolescent idiopathic scoliosis: A systematic review and genetic meta-analysis. Revista Brasileira de Ortopedia, 55(1), 8-16. https://doi. org/10. 1016/j. rboe. 2018. 03. 001
[8]. Liang, Z. -T. , et al. (2021). The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 35(9), e21839. https://doi. org/10. 1096/fj. 202100759R
[9]. Ahmadi, S. , et al. (2018). Relationship between estrogen and body composition, energy, and endocrine factors in obese women with normal and low REE. Steroids, 130, 31-35. https://doi. org/10. 1016/j. steroids. 2017. 12. 008
[10]. Kotwicki, T. , et al. (2022). Estrogen receptor type 1 and type 2 presence in paravertebral skeletal muscles: Expression level and relation to phenotype in children with idiopathic scoliosis. Genes, 13(5), 739. https://doi. org/10. 3390/genes13050739
[11]. Liu, Z. , et al. (2015). Polymorphism of rs2767485 in leptin receptor gene is associated with the occurrence of adolescent idiopathic scoliosis. Spine, 40(20), 1593-1598. https://doi. org/10. 1097/BRS. 0000000000001095
[12]. Clark, E. M. , et al. (2014). Association between components of body composition and scoliosis: A prospective cohort study reporting differences identifiable before the onset of scoliosis. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 29(8), 1729-1736. https://doi. org/10. 1002/jbmr. 2207
[13]. Quinton, N. D. , et al. (1999). Leptin binding activity changes with age: The link between leptin and puberty. The Journal of Clinical Endocrinology & Metabolism, 84(7), 2336-2341. https://doi. org/10. 1210/jcem. 84. 7. 5834
[14]. Yang, Y. , et al. (2023). Association between age at menarche and bone mineral density in postmenopausal women. Journal of Orthopaedic Surgery and Research, 18(1), 51. https://doi. org/10. 1186/s13018-023-03520-2
[15]. Rostami, S. , et al. (2015). The LEP G-2548A gene polymorphism is associated with age at menarche and breast cancer susceptibility. Gene, 557(2), 154-157. https://doi. org/10. 1016/j. gene. 2014. 12. 021
[16]. Thillard, M. J. (1959). Déformations de la colonne vertébrale consécutives à l’épiphysectomie chez le poussin [Vertebral column deformities following epiphysectomy in the chick]. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, 248(8), 1238-1240.
[17]. Goultidis, T. T. , et al. (2014). Higher levels of melatonin in early stages of adolescent idiopathic scoliosis: Toward a new scenario. Journal of Pediatric Orthopedics, 34(8), 768-773. https://doi. org/10. 1097/BPO. 0000000000000207
[18]. Gargano, G. , et al. (2022). Melatonin and adolescent idiopathic scoliosis: The present evidence. The Surgeon: Journal of the Royal Colleges of Surgeons of Edinburgh and Ireland, 20(6), e315-e321. https://doi. org/10. 1016/j. surge. 2021. 07. 008
[19]. Qiu, S. , et al. (2020). Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. Life Sciences, 248, 117455. https://doi. org/10. 1016/j. lfs. 2020. 117455
[20]. Wang, C. , & Wu, S. (2023). The application of endocrine hormones in adolescent idiopathic scoliosis. Chinese Journal of School Health, 44(08), 1276-1280. https://doi. org/10. 16835/j. cnki. 1000-9817. 2023. 08. 035
[21]. Lim, J. W. , et al. (2024). Association between changes in menarcheal age and adolescent idiopathic scoliosis: An analysis of 38, 879 patients over 20 years. Clinics in Orthopedic Surgery, 16(5), 807-812. https://doi. org/10. 4055/cios23336
[22]. Tsinopoulou, V. R. , et al. (2025). Genetic determinants of age at menarche: Does the LIN28B gene play a role? A narrative review. Hormones (Athens, Greece), 24(1), 167-177. https://doi. org/10. 1007/s42000-024-00594-3
[23]. Roberts, S. A. , & Kaiser, U. B. (2020). Genetics in endocrinology: Genetic etiologies of central precocious puberty and the role of imprinted genes. European Journal of Endocrinology, 183(4), R107-R117. https://doi. org/10. 1530/EJE-20-0103
[24]. Feigelson, H. S. , et al. (1997). A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Research, 57(6), 1063-1065.
[25]. Pei, Y. F. , et al. (2008). CYP17 MspA1 polymorphism and age at menarche: A meta-analysis. Disease Markers, 25(2), 87-95. https://doi. org/10. 1155/2008/403982
[26]. Goodbody, C. M. , et al. (2017). Presentation of adolescent idiopathic scoliosis: The bigger the kid, the bigger the curve. Journal of Pediatric Orthopedics, 37(1), 41-46. https://doi. org/10. 1097/BPO. 0000000000000580
[27]. Chiurazzi, M. , et al. (2020). Impact of genetic variations and epigenetic mechanisms on the risk of obesity. International Journal of Molecular Sciences, 21(23), 9035. https://doi. org/10. 3390/ijms21239035
[28]. Saeed, S. , et al. (2015). Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity (Silver Spring, Md. ), 23(8), 1687-1695. https://doi. org/10. 1002/oby. 21142
[29]. Zou, Y. , et al. (2022). The prevalence of scoliosis screening positive and its influencing factors: A school-based cross-sectional study in Zhejiang Province, China. Frontiers in Public Health, 10, 773594. https://doi. org/10. 3389/fpubh. 2022. 773594
[30]. Margalit, A. , et al. (2017). Body mass hides the curve: Thoracic scoliometer readings vary by body mass index value. Journal of Pediatric Orthopedics, 37(4), e255-e260. https://doi. org/10. 1097/BPO. 0000000000000899
[31]. Scaturro, D. , et al. (2022). Is there a relationship between idiopathic scoliosis and body mass? A scoping review. Nutrients, 14(19), 4011. https://doi. org/10. 3390/nu14194011
[32]. Durmala, J. , et al. (2013). The usefulness of bioelectrical body composition analysis (BIA) in the proper assessment of nutritional status in children and adolescents with idiopathic scoliosis (IS). Scoliosis, 8(S2), O35.
[33]. Matusik, E. , et al. (2016). Association of body composition with curve severity in children and adolescents with idiopathic scoliosis (IS). Nutrients, 8(2), 71. https://doi. org/10. 3390/nu8020071
[34]. Miyagi, M. , et al. (2020). Body composition in Japanese girls with adolescent idiopathic scoliosis. Spine Surgery and Related Research, 5(2), 68-74. https://doi. org/10. 22603/ssrr. 2020-0088
[35]. Tam, E. M. S. , et al. (2016). Lower muscle mass and body fat in adolescent idiopathic scoliosis are associated with abnormal leptin bioavailability. Spine, 41(11), 940-946. https://doi. org/10. 1097/BRS. 0000000000001376
[36]. Wang, W. , et al. (2016). Body composition in males with adolescent idiopathic scoliosis: A case-control study with dual-energy X-ray absorptiometry. BMC Musculoskeletal Disorders, 17, 107. https://doi. org/10. 1186/s12891-016-0968-0
[37]. Glavaš, J. , et al. (2023). The impact of physical activity on adolescent idiopathic scoliosis. Life (Basel, Switzerland), 13(5), 1180. https://doi. org/10. 3390/life13051180
[38]. Tobias, J. H. , et al. (2019). Association between physical activity and scoliosis: A prospective cohort study. International Journal of Epidemiology, 48(4), 1152-1160. https://doi. org/10. 1093/ije/dyy268
[39]. Qi, X. , et al. (2023). Correlation between physical activity and adolescent idiopathic scoliosis: A systematic review. BMC Musculoskeletal Disorders, 24(1), 978. https://doi. org/10. 1186/s12891-023-07114-1
[40]. Lv, Y. Z. (2024). Research on the effects of exercise intervention on ATR and physical fitness in elementary school students with idiopathic scoliosis. (Master’s thesis). Wuhan Sports University. https://doi. org/10. 27384/d. cnki. gwhtc. 2024. 000046
[41]. Mousavi, L. , et al. (2022). Prevalence of idiopathic scoliosis in athletes: A systematic review and meta-analysis. BMJ Open Sport & Exercise Medicine, 8(3), e001312. https://doi. org/10. 1136/bmjsem-2022-001312
[42]. Hell, A. K. , et al. (2021). Einfluss des Schulrucksackgewichtes bei Grundschulkindern: Gang, Muskelaktivität, Haltung und Stabilität [The influence of backpack weight in school children: Gait, muscle activity, posture, and stability]. Der Orthopade, 50(6), 446-454. https://doi. org/10. 1007/s00132-020-04047-8
[43]. Presta, V. , et al. (2020). One-shoulder carrying school backpack strongly affects gait swing phase and pelvic tilt: A case study. Acta Bio-Medica: Atenei Parmensis, 91(3-S), 168-170. https://doi. org/10. 23750/abm. v91i3-S. 9435
[44]. Wajchenberg, M. , et al. (2015). Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: A cross-sectional study. Medicine, 94(8), e598. https://doi. org/10. 1097/MD. 0000000000000598
[45]. Burner, W. L. , 3rd, et al. (1982). Osteoporosis and acquired back deformities. Journal of Pediatric Orthopedics, 2(4), 383-385. https://doi. org/10. 1097/01241398-198210000-00006
[46]. Li, X. , et al. (2020). Persistent low-normal bone mineral density in adolescent idiopathic scoliosis with different curve severity: A longitudinal study from presentation to beyond skeletal maturity and peak bone mass. Bone, 133, 115217. https://doi. org/10. 1016/j. bone. 2019. 115217
[47]. Korkmaz, H. A. , & Özkan, B. (2022). Impact of obesity on bone metabolism in children. Journal of Pediatric Endocrinology & Metabolism: JPEM, 35(5), 557-565. https://doi. org/10. 1515/jpem-2021-0714
[48]. Lam, T. P. , et al. (2021). A six-year longitudinal cohort study on the changes in bone density and bone quality up to peak bone mass in adolescent idiopathic scoliosis (AIS) with and without 2 years of calcium and Vitamin D supplementation. Studies in Health Technology and Informatics, 280, 31-34. https://doi. org/10. 3233/SHTI210429
[49]. Yuan, Y. , et al. (2016). The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Progress in Biophysics and Molecular Biology, 122(2), 122-130. https://doi. org/10. 1016/j. pbiomolbio. 2015. 11. 005
[50]. Guan, Q. , et al. (2021). Mechanisms of melatonin in obesity: A review. International Journal of Molecular Sciences, 23(1), 218. https://doi. org/10. 3390/ijms23010218
Cite this article
Liu,Y. (2025). Analysis of Susceptibility Factors for Idiopathic Scoliosis in Children and Adolescents. Theoretical and Natural Science,117,39-49.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Catanzariti, J. -F. , et al. (2023). Idiopathic adolescent scoliosis and obesity: Prevalence study. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 32(6), 2196-2202. https://doi. org/10. 1007/s00586-023-07709-1
[2]. Wong, A. Y. L. , et al. (2019). How common is back pain and what biopsychosocial factors are associated with back pain in patients with adolescent idiopathic scoliosis? Clinical Orthopaedics and Related Research, 477(4), 676-686. https://doi. org/10. 1097/CORR. 0000000000000569
[3]. Mitsiaki, I. , et al. (2022). Adolescent idiopathic scoliosis and mental health disorders: A narrative review of the literature. Children (Basel, Switzerland), 9(5), 597. https://doi. org/10. 3390/children9050597
[4]. Cheng, T. , et al. (2022). Idiopathic scoliosis: A systematic review and meta-analysis of heritability. EFORT Open Reviews, 7(6), 414-421. https://doi. org/10. 1530/EOR-22-0026
[5]. Dou, Q. , et al. (2023). Academic-related factors and daily lifestyle habits associated with adolescent idiopathic scoliosis: A case-control study. Environmental Health and Preventive Medicine, 28, 23. https://doi. org/10. 1265/ehpm. 22-00243
[6]. Watanabe, K. , et al. (2017). Physical activities and lifestyle factors related to adolescent idiopathic scoliosis. The Journal of Bone and Joint Surgery. American Volume, 99(4), 284-294. https://doi. org/10. 2106/JBJS. 16. 00459
[7]. Sobhan, M. R. , et al. (2020). Association of ESRα XbaI A > G, ESRα PvuII T > C and ESRβ AlwNI T > C polymorphisms with the risk of developing adolescent idiopathic scoliosis: A systematic review and genetic meta-analysis. Revista Brasileira de Ortopedia, 55(1), 8-16. https://doi. org/10. 1016/j. rboe. 2018. 03. 001
[8]. Liang, Z. -T. , et al. (2021). The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 35(9), e21839. https://doi. org/10. 1096/fj. 202100759R
[9]. Ahmadi, S. , et al. (2018). Relationship between estrogen and body composition, energy, and endocrine factors in obese women with normal and low REE. Steroids, 130, 31-35. https://doi. org/10. 1016/j. steroids. 2017. 12. 008
[10]. Kotwicki, T. , et al. (2022). Estrogen receptor type 1 and type 2 presence in paravertebral skeletal muscles: Expression level and relation to phenotype in children with idiopathic scoliosis. Genes, 13(5), 739. https://doi. org/10. 3390/genes13050739
[11]. Liu, Z. , et al. (2015). Polymorphism of rs2767485 in leptin receptor gene is associated with the occurrence of adolescent idiopathic scoliosis. Spine, 40(20), 1593-1598. https://doi. org/10. 1097/BRS. 0000000000001095
[12]. Clark, E. M. , et al. (2014). Association between components of body composition and scoliosis: A prospective cohort study reporting differences identifiable before the onset of scoliosis. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 29(8), 1729-1736. https://doi. org/10. 1002/jbmr. 2207
[13]. Quinton, N. D. , et al. (1999). Leptin binding activity changes with age: The link between leptin and puberty. The Journal of Clinical Endocrinology & Metabolism, 84(7), 2336-2341. https://doi. org/10. 1210/jcem. 84. 7. 5834
[14]. Yang, Y. , et al. (2023). Association between age at menarche and bone mineral density in postmenopausal women. Journal of Orthopaedic Surgery and Research, 18(1), 51. https://doi. org/10. 1186/s13018-023-03520-2
[15]. Rostami, S. , et al. (2015). The LEP G-2548A gene polymorphism is associated with age at menarche and breast cancer susceptibility. Gene, 557(2), 154-157. https://doi. org/10. 1016/j. gene. 2014. 12. 021
[16]. Thillard, M. J. (1959). Déformations de la colonne vertébrale consécutives à l’épiphysectomie chez le poussin [Vertebral column deformities following epiphysectomy in the chick]. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, 248(8), 1238-1240.
[17]. Goultidis, T. T. , et al. (2014). Higher levels of melatonin in early stages of adolescent idiopathic scoliosis: Toward a new scenario. Journal of Pediatric Orthopedics, 34(8), 768-773. https://doi. org/10. 1097/BPO. 0000000000000207
[18]. Gargano, G. , et al. (2022). Melatonin and adolescent idiopathic scoliosis: The present evidence. The Surgeon: Journal of the Royal Colleges of Surgeons of Edinburgh and Ireland, 20(6), e315-e321. https://doi. org/10. 1016/j. surge. 2021. 07. 008
[19]. Qiu, S. , et al. (2020). Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. Life Sciences, 248, 117455. https://doi. org/10. 1016/j. lfs. 2020. 117455
[20]. Wang, C. , & Wu, S. (2023). The application of endocrine hormones in adolescent idiopathic scoliosis. Chinese Journal of School Health, 44(08), 1276-1280. https://doi. org/10. 16835/j. cnki. 1000-9817. 2023. 08. 035
[21]. Lim, J. W. , et al. (2024). Association between changes in menarcheal age and adolescent idiopathic scoliosis: An analysis of 38, 879 patients over 20 years. Clinics in Orthopedic Surgery, 16(5), 807-812. https://doi. org/10. 4055/cios23336
[22]. Tsinopoulou, V. R. , et al. (2025). Genetic determinants of age at menarche: Does the LIN28B gene play a role? A narrative review. Hormones (Athens, Greece), 24(1), 167-177. https://doi. org/10. 1007/s42000-024-00594-3
[23]. Roberts, S. A. , & Kaiser, U. B. (2020). Genetics in endocrinology: Genetic etiologies of central precocious puberty and the role of imprinted genes. European Journal of Endocrinology, 183(4), R107-R117. https://doi. org/10. 1530/EJE-20-0103
[24]. Feigelson, H. S. , et al. (1997). A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Research, 57(6), 1063-1065.
[25]. Pei, Y. F. , et al. (2008). CYP17 MspA1 polymorphism and age at menarche: A meta-analysis. Disease Markers, 25(2), 87-95. https://doi. org/10. 1155/2008/403982
[26]. Goodbody, C. M. , et al. (2017). Presentation of adolescent idiopathic scoliosis: The bigger the kid, the bigger the curve. Journal of Pediatric Orthopedics, 37(1), 41-46. https://doi. org/10. 1097/BPO. 0000000000000580
[27]. Chiurazzi, M. , et al. (2020). Impact of genetic variations and epigenetic mechanisms on the risk of obesity. International Journal of Molecular Sciences, 21(23), 9035. https://doi. org/10. 3390/ijms21239035
[28]. Saeed, S. , et al. (2015). Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity (Silver Spring, Md. ), 23(8), 1687-1695. https://doi. org/10. 1002/oby. 21142
[29]. Zou, Y. , et al. (2022). The prevalence of scoliosis screening positive and its influencing factors: A school-based cross-sectional study in Zhejiang Province, China. Frontiers in Public Health, 10, 773594. https://doi. org/10. 3389/fpubh. 2022. 773594
[30]. Margalit, A. , et al. (2017). Body mass hides the curve: Thoracic scoliometer readings vary by body mass index value. Journal of Pediatric Orthopedics, 37(4), e255-e260. https://doi. org/10. 1097/BPO. 0000000000000899
[31]. Scaturro, D. , et al. (2022). Is there a relationship between idiopathic scoliosis and body mass? A scoping review. Nutrients, 14(19), 4011. https://doi. org/10. 3390/nu14194011
[32]. Durmala, J. , et al. (2013). The usefulness of bioelectrical body composition analysis (BIA) in the proper assessment of nutritional status in children and adolescents with idiopathic scoliosis (IS). Scoliosis, 8(S2), O35.
[33]. Matusik, E. , et al. (2016). Association of body composition with curve severity in children and adolescents with idiopathic scoliosis (IS). Nutrients, 8(2), 71. https://doi. org/10. 3390/nu8020071
[34]. Miyagi, M. , et al. (2020). Body composition in Japanese girls with adolescent idiopathic scoliosis. Spine Surgery and Related Research, 5(2), 68-74. https://doi. org/10. 22603/ssrr. 2020-0088
[35]. Tam, E. M. S. , et al. (2016). Lower muscle mass and body fat in adolescent idiopathic scoliosis are associated with abnormal leptin bioavailability. Spine, 41(11), 940-946. https://doi. org/10. 1097/BRS. 0000000000001376
[36]. Wang, W. , et al. (2016). Body composition in males with adolescent idiopathic scoliosis: A case-control study with dual-energy X-ray absorptiometry. BMC Musculoskeletal Disorders, 17, 107. https://doi. org/10. 1186/s12891-016-0968-0
[37]. Glavaš, J. , et al. (2023). The impact of physical activity on adolescent idiopathic scoliosis. Life (Basel, Switzerland), 13(5), 1180. https://doi. org/10. 3390/life13051180
[38]. Tobias, J. H. , et al. (2019). Association between physical activity and scoliosis: A prospective cohort study. International Journal of Epidemiology, 48(4), 1152-1160. https://doi. org/10. 1093/ije/dyy268
[39]. Qi, X. , et al. (2023). Correlation between physical activity and adolescent idiopathic scoliosis: A systematic review. BMC Musculoskeletal Disorders, 24(1), 978. https://doi. org/10. 1186/s12891-023-07114-1
[40]. Lv, Y. Z. (2024). Research on the effects of exercise intervention on ATR and physical fitness in elementary school students with idiopathic scoliosis. (Master’s thesis). Wuhan Sports University. https://doi. org/10. 27384/d. cnki. gwhtc. 2024. 000046
[41]. Mousavi, L. , et al. (2022). Prevalence of idiopathic scoliosis in athletes: A systematic review and meta-analysis. BMJ Open Sport & Exercise Medicine, 8(3), e001312. https://doi. org/10. 1136/bmjsem-2022-001312
[42]. Hell, A. K. , et al. (2021). Einfluss des Schulrucksackgewichtes bei Grundschulkindern: Gang, Muskelaktivität, Haltung und Stabilität [The influence of backpack weight in school children: Gait, muscle activity, posture, and stability]. Der Orthopade, 50(6), 446-454. https://doi. org/10. 1007/s00132-020-04047-8
[43]. Presta, V. , et al. (2020). One-shoulder carrying school backpack strongly affects gait swing phase and pelvic tilt: A case study. Acta Bio-Medica: Atenei Parmensis, 91(3-S), 168-170. https://doi. org/10. 23750/abm. v91i3-S. 9435
[44]. Wajchenberg, M. , et al. (2015). Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: A cross-sectional study. Medicine, 94(8), e598. https://doi. org/10. 1097/MD. 0000000000000598
[45]. Burner, W. L. , 3rd, et al. (1982). Osteoporosis and acquired back deformities. Journal of Pediatric Orthopedics, 2(4), 383-385. https://doi. org/10. 1097/01241398-198210000-00006
[46]. Li, X. , et al. (2020). Persistent low-normal bone mineral density in adolescent idiopathic scoliosis with different curve severity: A longitudinal study from presentation to beyond skeletal maturity and peak bone mass. Bone, 133, 115217. https://doi. org/10. 1016/j. bone. 2019. 115217
[47]. Korkmaz, H. A. , & Özkan, B. (2022). Impact of obesity on bone metabolism in children. Journal of Pediatric Endocrinology & Metabolism: JPEM, 35(5), 557-565. https://doi. org/10. 1515/jpem-2021-0714
[48]. Lam, T. P. , et al. (2021). A six-year longitudinal cohort study on the changes in bone density and bone quality up to peak bone mass in adolescent idiopathic scoliosis (AIS) with and without 2 years of calcium and Vitamin D supplementation. Studies in Health Technology and Informatics, 280, 31-34. https://doi. org/10. 3233/SHTI210429
[49]. Yuan, Y. , et al. (2016). The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Progress in Biophysics and Molecular Biology, 122(2), 122-130. https://doi. org/10. 1016/j. pbiomolbio. 2015. 11. 005
[50]. Guan, Q. , et al. (2021). Mechanisms of melatonin in obesity: A review. International Journal of Molecular Sciences, 23(1), 218. https://doi. org/10. 3390/ijms23010218









