1. Introduction
Inflammatory bowel disease (IBD) is generally divided into ulcerative colitis and Crohn's disease. It is a systemic disease with symptoms that occur not only in the gastrointestinal tract but also in other organs. In recent years, the incidence and hospitalization rates of IBD in emerging industrialized countries have continued to rise, reflecting that IBD has gradually become an important international public health issue [1]. However, existing conventional treatments such as surgery, Mesalazine, and immunosuppressants cannot cure it, but only control inflammation and prevent complications [2-3]. In recent years, anti-TNF biologics such as subcutaneous or intravenous infliximab and their generics have been shown to be effective for moderate or severe IBD [4]. However, to achieve equality and affordability in the management of IBD worldwide, innovation in the reliance on biologics is needed [5-6]. New treatments, such as Chinese herbal medicine and intestinal flora regulation, have been shown to be effective for IBD [7-8]. Nanoparticles have the potential to protect drugs and promote targeted drug transport to improve efficacy. Previous studies have shown that delivering drugs or microorganisms through nanomaterials, or directly using plant nanoparticles, has significant effect on IBD [9-11].
This article reviews the recent progress in nano formulations for the treatment of inflammatory bowel disease and summarizes the current challenges of nanoparticles in the treatment of IBD and future perspectives. This study provides new ideas and suggestions for the follow-up treatment of inflammatory bowel disease.
2. Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease is divided into ulcerative colitis (UC) and Crohn’s disease (CD). Two subtypes differ mainly from symptoms and affected regions. UC is a lifelong disease, usually characterized by relapses and remissions [3]. It is generally believed that the most accurate diagnostic method for UC is endoscopic biopsy [12]. Some biomarkers such as fecal leukocyte esterase and C-reactive protein-to-bilirubin are also considered to have good reference value for the diagnosis of UC [13-14]. The typical endoscopic features are the gradual disappearance of vascular structures, and the mucosa changes from erythema to blood adhesion until spontaneous bleeding and ulcers occur [3]. CD often affects the ileum and colon in a discontinuous, patchy, segmental, and transmural manner, but can also affect any part of the digestive tract [15]. Endoscopy and histopathology reveal cobblestone lesions in the colon that are only seen in CD and are therefore the best means of differentiation [16]. CD often affects the ileum and colon in a discontinuous, patchy, segmental, and transmural manner, but can also affect any part of the digestive tract [15]. Endoscopy and histopathology reveal cobblestone lesions in the colon that are only seen in CD and are therefore the best means of differentiation [16].
It is generally believed that the etiology of IBD is multifactorial [15]. In addition to common genetic susceptibility site variants that have been shown to contribute less to disease susceptibility, damage to the intestinal epithelial barrier is a key cause [15, 17]. At the same time, single nucleotide polymorphisms of genes encoding cytokines, cytokine receptors, cytokine-induced transcription factors, cytokine downstream signaling molecules, etc. have also been shown to be associated with the early onset of IBD [18]. In addition, failure of microbial recognition and phagocytosis mechanisms mediated by NOD-like cytoplasmic receptors (NLRs) and Toll-like receptors (TLRs) can cause intestinal microbial dysbiosis, leading to the invasion of bacteria such as Clostridium innocua and Escherichia coli and the further progress of IBD [19-20].
3. Some nanoparticle delivery system for IBD
3.1. Drug delivery systems
3.1.1. Cytokine and receptor-related delivery systems
IL-10 is a key cytokine in intestinal inflammation and has shown encouraging therapeutic effects in animal models of colitis [21]. Liu et al. used Escherichia coli Top10 carrying a mouse IL-10 gene plasmid to transfect human embryonic kidney 293T cells (HEK293T) and then ultracentrifuged them. The supernatant was analyzed for control to obtain IL-10-containing extracellular vesicles (IL10-EVs) with a diameter like that of normal cell extracellular vesicles [22]. Macrophage galactose C-type lectin (MGL) is a substance inducing colonic lamina propria macrophages to produce IL-10, recognizing galactose (Gal) to exert its effect [23-24]. Therefore, Liu et al. coupled Gal with 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Polyethyleneglycol2000-Amino (DSPE-PEG-NH2) with a targeting moiety to generate DSPE-PEG-Gal and inserted it into IL10-EVs to obtain Gal-IL10-EVs. In vitro studies showed the expression of inflammatory factors in macrophages treated with Gal-IL10-EVs was downregulated in a dose-dependent manner, reflecting the possible therapeutic effect of this delivery system on colitis [22].
3.1.2. Metal nanoparticle-related delivery systems
Cerium dioxide is noted for its unique immunomodulatory properties and enzyme mimetic activity that protects tissues from reactive oxygen species (ROS) overproduction and inflammation [25]. Min et al. developed an oral inflamed colon-targeted nanotherapeutics (ICAN), which loaded cerium dioxide nanoparticles (CeNPs) onto mesoporous silica nanoparticles (MSNs) and coated them with negatively charged polyacrylic acid (PAA). In vitro experiments showed that it had a high ROS clearance rate in artificial gastrointestinal fluid. In vivo researches showed that it could regulate the level of oxidative stress in the lesion site, as illustrated in Figure 1 [26].
Since the antioxidant mechanism of ceria itself has energy defects, Li et al. synthesized ceria nanoparticles embedded with gold nanoparticles (Au/CeO2) and coated them with hyaluronic acid (HA) to obtain Au/CeO2@HA. The porous structure of Au/CeO2@HA increases the contact area and improves the antioxidant activity. Figure 2 shows that it can be accumulated in the inflamed colon tissue through oral administration to reduce colon damage, which to some extent makes up for the shortcomings of ceria itself [27].
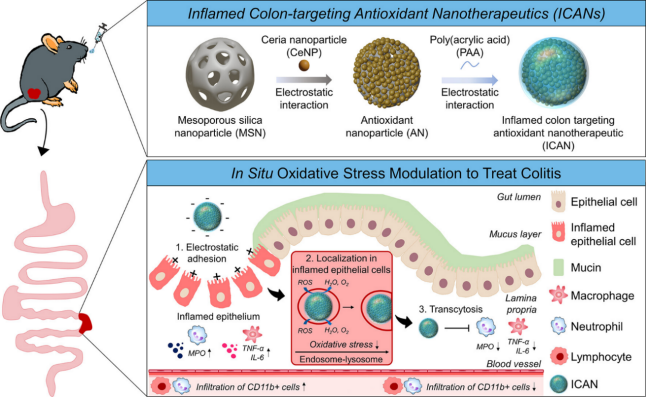
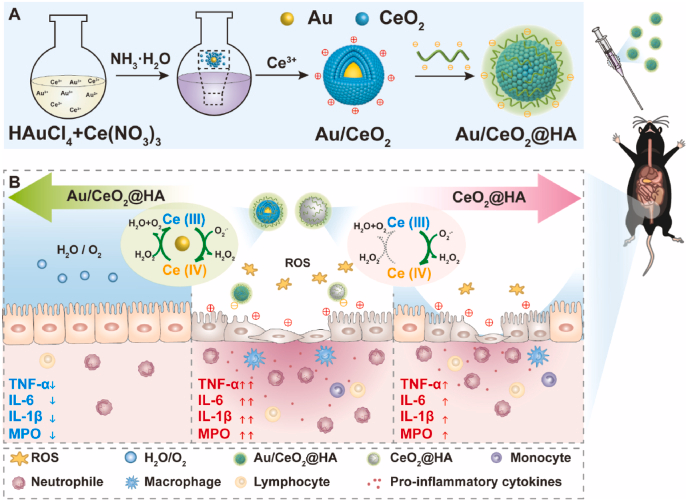
3.2. Plant related delivery systems
3.2.1. Plant exosome related delivery system
Exosomes are nanoscale lipid bilayer vesicles that accurately deliver particles of various sizes to inflammatory areas [28]. Edible plant-derived exosome-like nanoparticles (PDENs) offer benefits over animal-derived ones, such as oral therapeutic efficacy, non-toxicity, and low immunogenicity [29]. Yang et al. isolated ginseng-derived nanoparticles (GDNP) from ginseng root juice by differential centrifugation. After purification and characterization, they were gavaged into C57BL/6J male mice modeled with dextran sulfate sodium (DSS). The results of vivo distribution and stability experimental analysis showed that GDNP was significantly absorbed in the gastrointestinal tract at all time points compared with other organs, as shown in Figure 3. At the same time, GDNP can reduce inflammatory responses and damage induced by intracellular toxins and DSS by activating the p62-Nrf2-Keap1 pathway, downregulating the proinflammatory factors, and blocking the Toll-like receptor 4 (TLR4)/MAPK pathway [11]. Kim et al.'s experiments demonstrated that the GDNP treatment significantly reduced the DSS-induced disease activity index in Balb/C mice. Notably, the long-term survival rate was nearly doubled that of the group using only phosphate buffered saline (PBS), as shown in Figure 4. These findings revealed the possible therapeutic mechanism and efficacy of GDNP for human IBD [30].
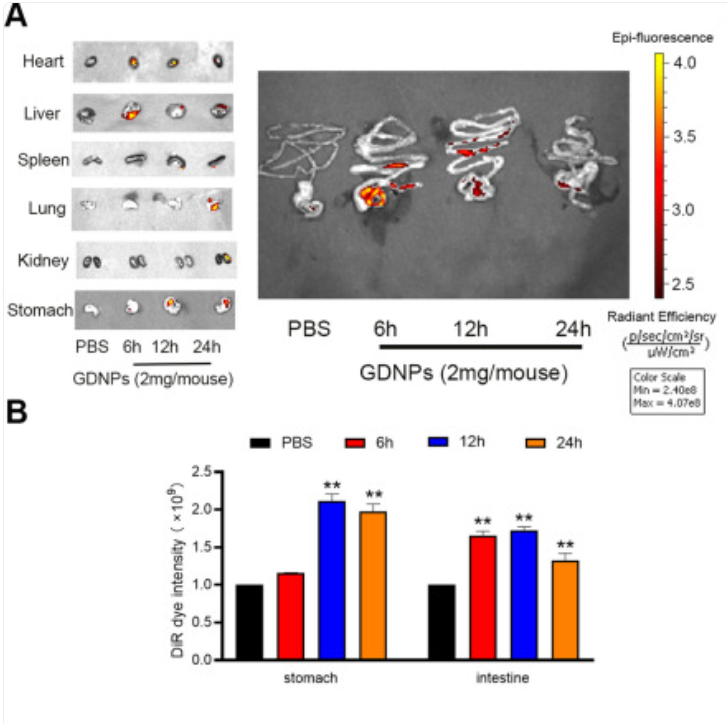
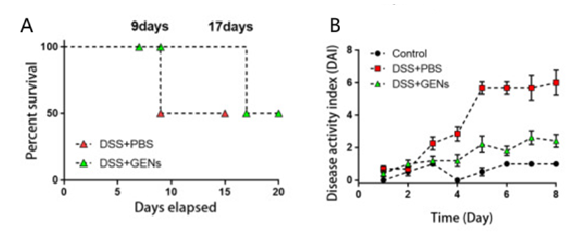
3.2.2. Plant component related delivery systems
Curcumin (CUR) is an polyphenol compound extracted from the rhizome of turmeric (Curcuma longa) with high pharmaceutical effects. As a promising candidate for the treatment of IBD, it can reduce oxidative stress damage by inhibiting NF-B activation, reducing TNF- expression levels, and improving intestinal damage by promoting the upregulation of anti-inflammatory factors such as IL-10 [31-32]. Although CUR is very beneficial, it still has problems such as low water solubility and poor metabolism [32]. To solve this problem, Lei et al. prepared nanoemulsions of curcumin and emodin (EMO), another anti-inflammatory plant ingredient with similar properties, and prepared an edible pH-sensitive colon-targeted delivery platform (CUR/EMO NE@SA) supported by a three-dimensional hydrogel network formed by sodium alginate (SA) and chitosan (CS). In vitro and in vivo experiments showed that the nanoemulsion had a high affinity for target cells and can release drug with change of microenvironment. When the concentration of CUR and EMO was 10g/mL, it significantly reduced TNF- and increased IL-10, exerting an anti-inflammatory effect. It also promoted the proliferation of caco-2 cells, indicating that it may also accelerate mucosal repair [33]. The nanoemulsion effectively improved the deficiencies of curcumin itself and revealed the feasibility of curcumin as an IBD drug.
4. Conclusion
As a global disease, inflammatory bowel disease has certain regional heterogeneity. Studies have found that the incidence of ulcerative colitis in Chinese cities is similar to that in Western countries, while the latter shown a higher rate of Crohn’s disease than the former. Some studies have also demonstrate that the higher incidence of Crohn's disease in Western countries than in Asians may be related to CD risk alleles. These studies reflect that the onset of inflammatory bowel disease is affected by multiple factors, and its causal relationship is difficult to determine. However, there are currently no nanoformulations that have been marketed and put into use for the treatment of IBD. According to theanalysis, the upcoming nanoformulations for the treatment of IBD should consider issues such as production scale, safety of long-term use, and personalized treatment strategies, and combine technologies such as microbiome regulation to develop more intelligent nanosystems while promoting clinical translation verification.
However, there are limitations in this study. For instance, the understanding of the underlying mechanisms and interactions between genetic and environmental factors remains to be explored. Future development should focus on integrating advanced technologies, such as microbiome modulation and personalized medicine, to create intelligent nanosystems that can better address individual patient needs and improve clinical outcomes.
References
[1]. Buie, M. J., et al. (2023). Global hospitalization trends for Crohn’s disease and ulcerative colitis in the 21st century: A systematic review with temporal analyses. Clinical Gastroenterology and Hepatology, 21(9), 2211-2221.
[2]. Lichtenstein, G. R., et al. (2018). ACG clinical guideline: Management of Crohn's disease in adults. Official Journal of the American College of Gastroenterology | ACG, 113(4).
[3]. Le Berre, C., Honap, S., & Peyrin-Biroulet, L. (2023). Ulcerative colitis. The Lancet, 402(10401), 571-584.
[4]. Schreiber, S., et al. (2021). Randomized controlled trial: Subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology, 160(7), 2340-2353.
[5]. Kaplan, G. G. (2015). The global burden of IBD: From 2015 to 2025. Nature Reviews Gastroenterology & Hepatology, 12(12), 720-727.
[6]. Ng, S. C., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. The Lancet, 390(10114), 2769-2778.
[7]. Wang, X., et al. (2024). The emerging role of the gut microbiota and its application in inflammatory bowel disease. Biomedicine & Pharmacotherapy, 179, 117302..
[8]. Zhu, M.-Z., et al. (2023). Exploring the efficacy of herbal medicinal products as oral therapy for inflammatory bowel disease. Biomedicine & Pharmacotherapy, 165, 115266.
[9]. Tang, X., et al. (2024). Targeted delivery of Fc-fused PD-L1 for effective management of acute and chronic colitis. Nature Communications, 15(1), 1673.
[10]. Hu, Q., et al. (2024). Polyphenolic nanoparticle-modified probiotics for microenvironment remodeling and targeted therapy of inflammatory bowel disease. ACS Nano, 18(20), 12917-12932.
[11]. Yang, S., et al. (2024). Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways. Journal of Nanobiotechnology, 22(1), 48.
[12]. Ishida, N., et al. (2021). C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Scientific Reports, 11(1), 12431.
[13]. Trasolini, R., et al. (2022). Fecal leukocyte esterase, an alternative biomarker to fecal calprotectin in inflammatory bowel disease: A pilot series. Gastro Hep Advances, 1(1), 45-51.
[14]. Huang, X., et al. (2023). Clinical significance of the C-reactive protein-to-bilirubin ratio in patients with ulcerative colitis. Frontiers in Medicine, 10.
[15]. Dolinger, M., Torres, J., & Vermeire, S. (2024). Crohn's disease. The Lancet, 403(10432), 1177-1191.
[16]. Annese, V., et al. (2013). European evidence-based consensus for endoscopy in inflammatory bowel disease. Journal of Crohn's and Colitis, 7(12), 982-1018.
[17]. Atreya, R., & Neurath, M. F. (2024). Biomarkers for personalizing IBD therapy: The quest continues. Clinical Gastroenterology and Hepatology, 22(7), 1353-1364.
[18]. Neurath, M. F. (2024). Strategies for targeting cytokines in inflammatory bowel disease. Nature Reviews Immunology, 24(8), 559-576.
[19]. Gilliland, A., et al. (2024). Pathobionts in inflammatory bowel disease: Origins, underlying mechanisms, and implications for clinical care. Gastroenterology, 166(1), 44-58.
[20]. Lopes, S. A., et al. (2023). Delivery strategies of probiotics from nano- and microparticles: Trends in the treatment of inflammatory bowel disease—An overview. Pharmaceutics, 15(11).
[21]. Li, M. C., & He, S. H. (2004). IL-10 and its related cytokines for treatment of inflammatory bowel disease. World Journal of Gastroenterology, 10(5), 620-625.
[22]. Liu, J., et al. (2023). Orally-delivered, cytokine-engineered extracellular vesicles for targeted treatment of inflammatory bowel disease. Small, 19(50), 2304023.
[23]. Fischer, S., et al. (2022). From structure to function - Ligand recognition by myeloid C-type lectin receptors. Computational and Structural Biotechnology Journal, 20, 5790-5812..
[24]. Saba, K., Denda-Nagai, K., & Irimura, T. (2009). A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. American Journal of Pathology, 174(1), 144-152..
[25]. Casals, E., et al. (2020). Cerium oxide nanoparticles: Advances in biodistribution, toxicity, and preclinical exploration. Small, 16(20), 1907322.
[26]. Min, D. K., et al. (2023). Orally administrated inflamed colon-targeted nanotherapeutics for inflammatory bowel disease treatment by oxidative stress level modulation in colitis. ACS Nano, 17(23), 24404-24416.
[27]. Li, M., et al. (2023). Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease. Bioactive Materials, 25, 95-106.
[28]. Wang, C., et al. (2023). Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian Journal of Pharmaceutical Sciences, 18(1), 100772.
[29]. Li, D. F., et al. (2023). Plant-derived exosomal nanoparticles: Potential therapeutic for inflammatory bowel disease. Nanoscale Advances, 5(14), 3575-3588.
[30]. Kim, J., et al. (2023). Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. Journal of Ginseng Research, 47(5), 627-637.
[31]. Karthikeyan, A., et al. (2021). Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceutics, 13(4).
[32]. Laurindo, L. F., et al. (2023). Curcumin-based nanomedicines in the treatment of inflammatory and immunomodulated diseases: An evidence-based comprehensive review. Pharmaceutics, 15(1).
[33]. Lei, F., et al. (2023). Oral hydrogel nanoemulsion co-delivery system treats inflammatory bowel disease via anti-inflammatory and promoting intestinal mucosa repair. Journal of Nanobiotechnology, 21(1), 275.
Cite this article
Ding,Y. (2025). Advances in Nanoparticles for Inflammatory Bowel Disease. Theoretical and Natural Science,131,1-7.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Interdisciplinary Frontiers in Pharmaceutical Sciences
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Buie, M. J., et al. (2023). Global hospitalization trends for Crohn’s disease and ulcerative colitis in the 21st century: A systematic review with temporal analyses. Clinical Gastroenterology and Hepatology, 21(9), 2211-2221.
[2]. Lichtenstein, G. R., et al. (2018). ACG clinical guideline: Management of Crohn's disease in adults. Official Journal of the American College of Gastroenterology | ACG, 113(4).
[3]. Le Berre, C., Honap, S., & Peyrin-Biroulet, L. (2023). Ulcerative colitis. The Lancet, 402(10401), 571-584.
[4]. Schreiber, S., et al. (2021). Randomized controlled trial: Subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology, 160(7), 2340-2353.
[5]. Kaplan, G. G. (2015). The global burden of IBD: From 2015 to 2025. Nature Reviews Gastroenterology & Hepatology, 12(12), 720-727.
[6]. Ng, S. C., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. The Lancet, 390(10114), 2769-2778.
[7]. Wang, X., et al. (2024). The emerging role of the gut microbiota and its application in inflammatory bowel disease. Biomedicine & Pharmacotherapy, 179, 117302..
[8]. Zhu, M.-Z., et al. (2023). Exploring the efficacy of herbal medicinal products as oral therapy for inflammatory bowel disease. Biomedicine & Pharmacotherapy, 165, 115266.
[9]. Tang, X., et al. (2024). Targeted delivery of Fc-fused PD-L1 for effective management of acute and chronic colitis. Nature Communications, 15(1), 1673.
[10]. Hu, Q., et al. (2024). Polyphenolic nanoparticle-modified probiotics for microenvironment remodeling and targeted therapy of inflammatory bowel disease. ACS Nano, 18(20), 12917-12932.
[11]. Yang, S., et al. (2024). Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways. Journal of Nanobiotechnology, 22(1), 48.
[12]. Ishida, N., et al. (2021). C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Scientific Reports, 11(1), 12431.
[13]. Trasolini, R., et al. (2022). Fecal leukocyte esterase, an alternative biomarker to fecal calprotectin in inflammatory bowel disease: A pilot series. Gastro Hep Advances, 1(1), 45-51.
[14]. Huang, X., et al. (2023). Clinical significance of the C-reactive protein-to-bilirubin ratio in patients with ulcerative colitis. Frontiers in Medicine, 10.
[15]. Dolinger, M., Torres, J., & Vermeire, S. (2024). Crohn's disease. The Lancet, 403(10432), 1177-1191.
[16]. Annese, V., et al. (2013). European evidence-based consensus for endoscopy in inflammatory bowel disease. Journal of Crohn's and Colitis, 7(12), 982-1018.
[17]. Atreya, R., & Neurath, M. F. (2024). Biomarkers for personalizing IBD therapy: The quest continues. Clinical Gastroenterology and Hepatology, 22(7), 1353-1364.
[18]. Neurath, M. F. (2024). Strategies for targeting cytokines in inflammatory bowel disease. Nature Reviews Immunology, 24(8), 559-576.
[19]. Gilliland, A., et al. (2024). Pathobionts in inflammatory bowel disease: Origins, underlying mechanisms, and implications for clinical care. Gastroenterology, 166(1), 44-58.
[20]. Lopes, S. A., et al. (2023). Delivery strategies of probiotics from nano- and microparticles: Trends in the treatment of inflammatory bowel disease—An overview. Pharmaceutics, 15(11).
[21]. Li, M. C., & He, S. H. (2004). IL-10 and its related cytokines for treatment of inflammatory bowel disease. World Journal of Gastroenterology, 10(5), 620-625.
[22]. Liu, J., et al. (2023). Orally-delivered, cytokine-engineered extracellular vesicles for targeted treatment of inflammatory bowel disease. Small, 19(50), 2304023.
[23]. Fischer, S., et al. (2022). From structure to function - Ligand recognition by myeloid C-type lectin receptors. Computational and Structural Biotechnology Journal, 20, 5790-5812..
[24]. Saba, K., Denda-Nagai, K., & Irimura, T. (2009). A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. American Journal of Pathology, 174(1), 144-152..
[25]. Casals, E., et al. (2020). Cerium oxide nanoparticles: Advances in biodistribution, toxicity, and preclinical exploration. Small, 16(20), 1907322.
[26]. Min, D. K., et al. (2023). Orally administrated inflamed colon-targeted nanotherapeutics for inflammatory bowel disease treatment by oxidative stress level modulation in colitis. ACS Nano, 17(23), 24404-24416.
[27]. Li, M., et al. (2023). Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease. Bioactive Materials, 25, 95-106.
[28]. Wang, C., et al. (2023). Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian Journal of Pharmaceutical Sciences, 18(1), 100772.
[29]. Li, D. F., et al. (2023). Plant-derived exosomal nanoparticles: Potential therapeutic for inflammatory bowel disease. Nanoscale Advances, 5(14), 3575-3588.
[30]. Kim, J., et al. (2023). Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. Journal of Ginseng Research, 47(5), 627-637.
[31]. Karthikeyan, A., et al. (2021). Curcumin and its modified formulations on inflammatory bowel disease (IBD): The story so far and future outlook. Pharmaceutics, 13(4).
[32]. Laurindo, L. F., et al. (2023). Curcumin-based nanomedicines in the treatment of inflammatory and immunomodulated diseases: An evidence-based comprehensive review. Pharmaceutics, 15(1).
[33]. Lei, F., et al. (2023). Oral hydrogel nanoemulsion co-delivery system treats inflammatory bowel disease via anti-inflammatory and promoting intestinal mucosa repair. Journal of Nanobiotechnology, 21(1), 275.









