1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder, and its core pathological features are manifested as abnormal deposition of beta-amyloid protein and excessive phosphorylation of tau protein. This disease is mainly characterized by progressive cognitive decline, accompanied by significant mental symptoms and behavioral abnormalities, including typical clinical phenotypes such as memory loss, disorientation, mood swings and decreased daily behavioral ability. Its pathogenesis is closely related to neuronal damage and synaptic dysfunction, and is currently regarded as the leading cause of dementia worldwide [1]. It affects over 50 million people worldwide. According to the "2023 China Alzheimer's Disease Data and Prevention and Control Strategies" research report, the current total number of AD patients in China has exceeded 10 million, and it shows significant age-related characteristics among the elderly population. Data shows that the proportion of AD patients among the elderly population aged 60 and above is close to 4% (3.9%), which highlights the urgency of the prevention and control of neurodegenerative diseases (NDDs) in the context of an aging society [2]. Therefore, it is of great significance to clarify the pathogenesis of AD and define the early diagnostic indicators and treatment plans of AD.
Exosomes are nanoscale (30-150 nm) membranous microvesicles that can mediate information transmission between neurons or/and glial cells to affect the development of the central nervous system, the regulation of synaptic activity, and the regeneration of damaged nerves [3,4]. When glial cells are in a state of stress, It secretes synaptophysin related to neural development [5], while EXO derived from microglia can enhance the metabolism of acrylamide and sphingosine in receptor neurons and increase the synthesis of neurotransmitters [6]. Under pathological conditions, EXO can participate in the diffusion of misfolded proteins and induce neuroinflammation and oxidative stress. EXO can affect various physiological and pathological processes by transmitting chemokines, misfolded proteins, antigens, microRNAs (miRNAs), and cytokines, etc [7,8].. Evidence indicates that EXO plays a significant role in and promotes the occurrence and development of the disease by regulating the pathological pathways of AD, which makes it a potential breakthrough diagnostic and therapeutic target. A comprehensive analysis of the multi-dimensional role of EXO in the pathogenic mechanism of AD, biomarker screening and intervention strategies is expected to lay a scientific foundation for the development of early precise diagnostic tools and targeted therapeutic technologies.
2. The pathological mechanism of AD
AD, as a highly prevalent NDD among the elderly population, accounts for 60% to 80% of all dementia cases. Its core pathological features are manifested as progressive cognitive decline and loss of autonomous living ability. Studies have confirmed that the molecular pathological mechanism of AD involves multiple pathway abnormalities: AD is characterized by the formation of senile plaques, which are caused by abnormal Aβ accumulation, and neurofibrillary tangles, which are caused by excessive phosphorylation of Tau protein. Chronic neuroinflammatory responses mediated by microglia, and oxidative stress damage caused by mitochondrial dysfunction. These pathological cascade reactions eventually lead to the destruction of synaptic plasticity, neuronal loss and neural network dysfunction through synergistic effects.
2.1. Aβ
One of the pathological markers of AD, Age spots are mainly composed of A short peptide containing 39 to 42 amino acids, namely β -amyloid protein (Aβ). Aβ is produced by the continuous cleavage of amyloid precursor protein (APP) by β -secretase and γ -secretase. At normal concentrations, Aβ has no toxicity and can play the role of A neuronal nutritional factor. It can also activate phosphokinase, regulate cholesterol transport, and inhibit excessive neuronal activation and other benefits. However, during the development of AD, some abnormalities of Aβ may occur, leading to its aggregation in brain tissue, which can cause a series of neural damages. Ultimately, it leads to neuronal dysfunction and neuronal death [9,10]. Injecting the Aβ fragment into the lateral ventricle or the CA1 area of the hippocampus can simulate the toxicity of Aβ to the central nervous system in AD patients. The length of the Aβ peptide can vary between 37 and 49 amino acids, and there are generally three types, namely Aβ 1-42, Aβ 1-40 and Aβ 25-35. Among them, Aβ 1-42 is the most dangerous form. Research has found that each oligomer has unique characteristics. The conformational differences of Aβ fibrils will have A fundamental impact on the detection of Aβ antibodies, which can occur in the brain of Aβ -induced AD models. Obvious Aβ deposition, reactive glial hyperplasia, oxidative stress, neuroinflammation, as well as synaptic and memory defects and other pathological manifestations of AD [11]
2.2. Tau protein
As the main pathological protein of AD, tau protein is currently the best biomarker of Cerebrospinal Fluid (CSF). Tau protein mainly includes total tau(t-tau) protein and p-tau protein. High expression of t-tau protein in CSF indicates the loss of cortical neurons. The expression of t-tau protein can effectively distinguish healthy people from AD. However, since the expression of t-tau protein in plasma is relatively low, whether it can be used as a plasma marker remains controversial. There are many types of p-tau proteins, including p-tau181, p-tau217, p-tau231 and p-tau235 proteins, which are widely used as biological markers of AD [12]. Excessive phosphorylation of Tau protein is another important pathological feature of AD. In normal nerve cells, tau protein can stabilize the microtubule system, promote the stability of nerve axons, and participate in protein transport and neuronal polarization. However, when Tau protein within neurons undergoes excessive phosphorylation, it leads to abnormal aggregation of nerve fibers in the cell body, axons, and dendrites of neurons, thereby generating neurofibrillary tangles (NFTS) . NFTs can further cause a decrease in the forward axoplasmic transport capacity, synaptic damage, and neuronal death.
3. EXO and AD
EXO transfer information and substances between cells and play multiple roles in the pathology of Alzheimer's disease (AD). Evidence indicates that EXO derived from neurons, on the one hand, promotes the production and oligomerization of Aβ, and on the other hand, acts as A carrier to transport Aβ and tau proteins to peripheral neurons. This dual effect eventually intensified the toxic effects of Aβ and tau proteins [13]. It's worth mentioning that EXO is involved in the degradation and clearance of Aβ. Autopsies and animal studies consistently demonstrate that EXO accumulates excessive levels of A in brain tissues [7].
In addition, the levels of miR-185-5p in EXO isolated from the brain tissues of AD model mice and the cultures of mouse cell blastoma Neuro-2a cells (N2a cells) with overexpression of APP were significantly decreased. EXO with A low miR-185-5p level can significantly increase the expression level of APP in recipient cells [13]. The above studies suggest that neuronal EXO can not only directly participate in the process of APP metabolism into Aβ, Moreover, it can indirectly participate in the metabolic process of APP through the transported miRNA. There is A large accumulation of EXO marker protein (ALIX) in the Aβ plaques of the brain tissue of AD patients, suggesting that EXO can not only transport Aβ but also participate in the formation of extracellular Aβ plaques [14]. In AD, EXO secreted by injured neurons can transport APP and Aβ to adjacent healthy neurons and accelerate the death of surrounding neurons. This leads to the spread of the pathological characteristics of AD. This leads to the spread of the pathological characteristics of AD. However, EXO secreted by neurons can also transport Aβ to the microglial lysosomes through transport mechanisms. to degrade Aβ. It is suggested that EXO in AD not only transcribes APP and Aβ to adjacent healthy neurons to accelerate the pathological process, but also acts as Aβ carrier to transport Aβ to microglia, which are dissolved by lysosomes (Figure 1) [14]. However, compared with normal mice, the uptake of EXO by microglia in the brain tissue of AD model mice is significantly reduced. It is suggested that the transport efficiency of EXO between neurons and microglia may be affected by the stage of disease development, and Microglia may have a role in deposition through EXO [15]. To sum up, EXO plays an important role in the production and degradation of Aβ, as well as extracellular transport and aggregation.
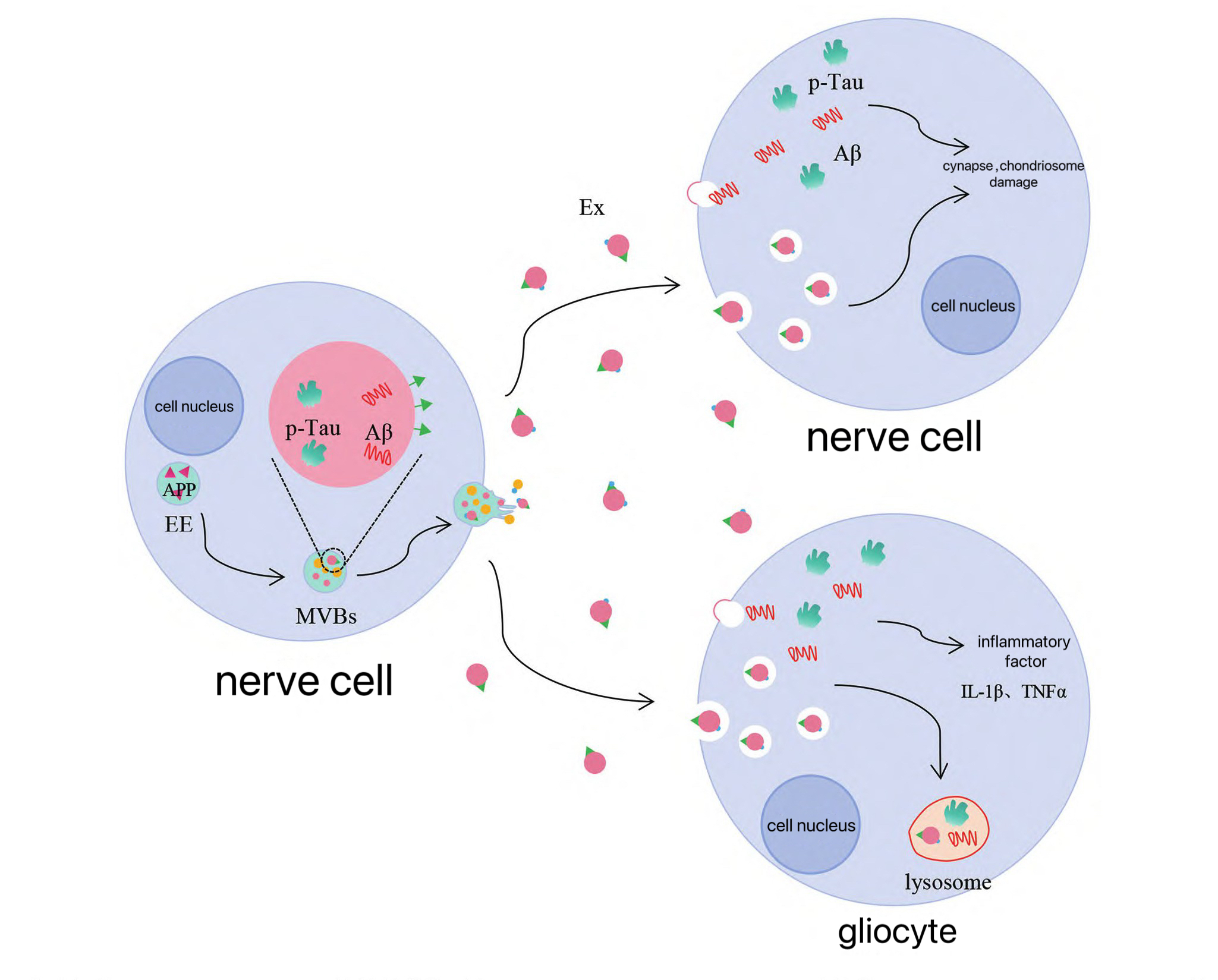
During the course of Alzheimer's disease, the level of EXOp-tau in cerebrospinal fluid shows a gradient increase from the early stage to mild/severe cases, suggesting that EXO may become a key regulatory factor promoting the early pathological evolution of the disease by mediating abnormal phosphorylation modification of tau protein and intracellular transport dysregulation [16] Human studies have shown that the EXO in the brain tissues of AD patients contains tau protein,It has a content that is much higher than that of normal people [8]. In the brain tissues of Alzheimer's disease, the tau protein seeds encapsulated in EXO not only demonstrate high efficiency in cross-neural circuit diffusion, but also avoid intracellular degradation pathways. Compared with the free tau protein, it significantly accelerates the formation rate of neurofibrillary tangles within neurons [8].
Studies have shown that EXO can not only mediate the transmission of tau protein between neurons through endocytosis and direct fusion of receptor cells, but also directly transmit across synapses to mediate the transfer of tau protein between neurons [17]. Animal studies have shown that EXO containing tau protein extracted from the plasma, cerebrospinal fluid or brain tissue of AD patients can induce the aggregation of tau protein in mouse neurons and microglia, and exhibit neurotoxic effects such as tau protein-induced neurofibrillary tangles [17]. Similarly, microglia can spread tau protein by secreting EXO. Inhibition of EXO synthesis can significantly reduce the diffusion of tau protein in vitro and in vivo [18]. Meanwhile, in the mouse model of tau protein brain diffusion, inhibition or elimination of microglia can also significantly inhibit the diffusion of tau protein from the entorhinal cortex to the dentate gyrus of the hippocampus and reduce the excitability of dentate gyrus neurons [18]. In conclusion, in the pathological process of AD, EXO drives the generation of its neurotoxic effects by regulating the abnormal aggregation and intercellular transmission of tau protein in neurons and glial cells.
4. Evaluation of the application value of EXO in the diagnosis and treatment of AD
Although EXO accelerates the occurrence and pathological process of AD by transporting Aβ and tau proteins, it also plays a protective role against AD to a certain extent. Neuron-derived EXO can transport Aβ and tau proteins to microglia and be taken up by microglia [13]. Microglia can clear the ingested Aβ and tau proteins to alleviate the pathological changes in patients with AD. In addition, exogenous EXO derived from various types of cells also shows a strong neuroprotective effect in AD. EXO derived from adipose-derived stem cells can effectively reduce The A levels that include Aβ 42, Aβ 40, and β 42/40 ratio. and apoptotic factors (p53, Bax, pro-caspase-3, cleaved-caspase-3) in in vitro AD model cells. Increasing the level of anti-apoptotic factor Bcl2 to exert neuroprotective effects [19], animal studies have shown that intranasal spray administration of human adipose-derived mesenchymal stem cells EXO can effectively reduce pathological Aβ deposition in AD model mice and improve synaptic plasticity, neurogenesis and learning and memory abilities of neurons in the hippocampal brain region of AD model mice. In addition, Phase I/II clinical studies have shown that after 12 weeks of intranasal spray treatment with allogeneic EXO mesenchymal stem cells from human adipose have been found to be effective in treating mild and moderate Alzheimer's disease., the degree of hippocampal atrophy was reduced and cognitive function was improved [20].
In addition, After injecting EXO derived from mesenchymal stem cells conjugated with central nervous system-specific rabies virus glycoprotein (RVG) peptide or EXO derived from human umbilical cord mesenchymal stem cells through the tail vein into APP/PS1 double transgenic AD model mice, the learning and memory ability of AD model mice in the water maze could be significantly improved. Increase the production levels of enzymes that break down A, like enkephalinase and insulin, and decrease the deposition and level of Aβ [21,22]. Meanwhile, injecting EXO derived from bone marrow mesenchymal stem cells into the lateral ventricle can reduce the expression levels of Aβ1-42 and p-Tau in the hippocampal brain region of AD model mice [23].
Neuroblastoma-derived EXO injected into the hippocampal brain region of AD model mice can capture Aβ and transport it to microglia for clearance, thereby reducing the level of Aβ in the hippocampal brain region, the formation of Aβ plaques, and Aβ -mediated synaptic injury [24]. In conclusion, endogenous EXO in the central nervous system can accelerate the clearance of Aβ and tau proteins through microglia. Exogenous mesenchymal stem cells and neuroblastoma-derived EXO can also reduce the production and aggregation of Aβ.
5. Conclusion
This article mainly focuses on the role of EXO in the pathological process of AD. As A carrier for information transmission and substance transport, EXO can transport APP and Aβ to healthy adjacent neurons, accelerating the spread of pathological characteristics of AD. However, EXO secreted by neurons can also transport Aβ to the lysosomes of microglia to degrade Aβ. It is suggested that EXO in AD will not only transport APP and Aβ to adjacent healthy neurons to accelerate the pathological process, but also act as A carrier to transport Aβ to microglia and dissolve it through lysosomes. EXO can not only mediate the transmission of tau protein between neurons through endocytosis and direct fusion of recipient cells, It can also be directly transmitted across neural synapses to mediate the transfer of tau protein between neurons. The spread of tau protein leads to neuronal dysfunction and further aggravates the pathological process of AD, demonstrating its important pathogenic role. I believe EXO has considerable potential in the field of AD, whether as A diagnostic marker or for the treatment of patients. If we can regulate the negative effect of EXO in transporting APP and Aβ to healthy neurons without affecting its transport of substances such as Aβ to microglia to promote their dissolution, I think it will be helpful for the treatment of AD.
References
[1]. Li, P., Quan, W., Wang, Z., Liu, Y., Cai, H., Chen, Y., ... and Zhou, Y., “Early-stage differentiation between Alzheimer’s disease and frontotemporal lobe degeneration: Clinical, neuropsychology, and neuroimaging features, ” Frontiers in Aging Neuroscience, vol. 14, 2022, Art. no. 981451.
[2]. Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., Li, Y., Li, Y., Zhu, M., Jiao, H., Song, Y., Shi, Y., Zhang, H., Gong, M., Wei, C., Tang, Y., Fang, B., Guo, D., Wang, F., Zhou, A., et al., “Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study, ” The Lancet Public Health, vol. 5, no. 12, pp. e661–e671, 2020.
[3]. Liu, Q., Tan, Y., Qu, T., Zhang, J., Duan, X., Xu, H., Mu, Y., Ma, H., and Wang, F., “Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro, ” Life Sciences, vol. 254, 2020, Art. no. 117772.
[4]. Caruso Bavisotto, C., Scalia, F., Marino Gammazza, A., Carlisi, D., Bucchieri, F., Conway de Macario, E., Macario, A. J. L., Cappello, F., and Campanella, C., “Extracellular vesicle-mediated cell–cell communication in the nervous system: Focus on neurological diseases, ” International Journal of Molecular Sciences, vol. 20, no. 2, p. 434, 2019.
[5]. Livne-Bar, I., Lam, S., Chan, D., Guo, X., Askar, I., Nahirnyj, A., Flanagan, J. G., and Sivak, J. M., “Pharmacologic inhibition of reactive gliosis blocks TNF-α-mediated neuronal apoptosis, ” Cell Death & Disease, vol. 7, no. 9, p. e2386, 2016.
[6]. Antonucci, F., Turola, E., Riganti, L., Caleo, M., Gabrielli, M., Perrotta, C., Novellino, L., Clementi, E., Giussani, P., Viani, P., Matteoli, M., and Verderio, C., “Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism, ” The EMBO Journal, vol. 31, no. 5, pp. 1231–1240, 2012.
[7]. Sardar Sinha, M., Ansell-Schultz, A., Civitelli, L., Hildesjö, C., Larsson, M., Lannfelt, L., Ingelsson, M., and Hallbeck, M., “Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers, ” Acta Neuropathologica, vol. 136, no. 1, pp. 41–56, 2018.
[8]. Ruan, Z., Pathak, D., Venkatesan Kalavai, S., Yoshii-Kitahara, A., Muraoka, S., Bhatt, N., Takamatsu-Yukawa, K., Hu, J., Wang, Y., Hersh, S., Ericsson, M., Gorantla, S., Gendelman, H. E., Kayed, R., Ikezu, S., Luebke, J. I., and Ikezu, T., “Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons, ” Brain, vol. 144, no. 1, pp. 288–309, 2021.
[9]. Craft, J. M., Watterson, D. M., and Van Eldik, L. J., “Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration, ” Glia, vol. 53, no. 5, pp. 484–490, 2006.
[10]. Manczak, M., Anekonda, T. S., Henson, E., Park, B. S., Quinn, J., and Reddy, P. H., “Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression, ” Human Molecular Genetics, vol. 15, no. 9, pp. 1437–1449, 2006.
[11]. Pei, J. Y., Liu, J. L., Liu, B., Dong, X. H., Cong, S. Y., Ren, X. M., and Zhou, Y. J., “Review of animal models of Alzheimer's disease applied in traditional Chinese medicine research, ” Chinese Journal of Comparative Medicine, pp. 1–14.
[12]. Mao, C. Y., Fang, R. H., and Cong, H., “Progress in the pathogenesis, diagnosis and treatment of Alzheimer’s disease, ” Jiangsu Medical Journal, vol. 51, no. 3, pp. 300–304, 2025.
[13]. Li, Y. X., Wang, Y. R., Liao, J. W., and Mou, L. W., “The role of exosomes in the pathogenesis, diagnosis and treatment of Alzheimer's disease, ” Life Sciences, vol. 37, no. 2, pp. 196–205, 2025.
[14]. Rajendran, L., Honsho, M., Zahn, T. R., Keller, P., Geiger, K. D., Verkade, P., and Simons, K., “Alzheimer’s disease beta-amyloid peptide is released in association with exosomes, ” Proceedings of the National Academy of Sciences of the United States of America, vol. 103, no. 30, pp. 11172–11177, 2006.
[15]. Zheng, T., Pu, J., Chen, Y., Mao, Y., Guo, Z., Pan, H., Zhang, L., Zhang, H., Sun, B., and Zhang, B., “Plasma exosomes spread and cluster around beta-amyloid plaques in an animal model of Alzheimer's disease, ” Frontiers in Aging Neuroscience, vol. 9, p. 12, 2017.
[16]. Saman, S., Kim, W., Raya, M., Visnick, Y., Miro, S., Saman, S., Jackson, B., McKee, A. C., Alvarez, V. E., Lee, N. C., and Hall, G. F., “Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease, ” The Journal of Biological Chemistry, vol. 287, no. 6, pp. 3842–3849, 2012.
[17]. Wang, Y., Balaji, V., Kaniyappan, S., Krüger, L., Irsen, S., Tepper, K., Chandupatla, R., Maetzler, W., Schneider, A., Mandelkow, E., and Mandelkow, E. M., “The release and trans-synaptic transmission of Tau via exosomes, ” Molecular Neurodegeneration, vol. 12, no. 1, p. 5, 2017.
[18]. Asai, H., Ikezu, S., Tsunoda, S., Medalla, M., Luebke, J., Haydar, T., Wolozin, B., Butovsky, O., Kügler, S., and Ikezu, T., “Depletion of microglia and inhibition of exosome synthesis halt tau propagation, ” Nature Neuroscience, vol. 18, no. 11, pp. 1584–1593, 2015.
[19]. Lee, M., Ban, J. J., Yang, S., Im, W., and Kim, M., “The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease, ” Brain Research, vol. 1691, pp. 87–93, 2018.
[20]. Ma, X., Huang, M., Zheng, M., Dai, C., Song, Q., Zhang, Q., Li, Q., Gu, X., Chen, H., Jiang, G., Yu, Y., Liu, X., Li, S., Wang, G., Chen, H., Lu, L., and Gao, X., “ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease, ” Journal of Controlled Release, vol. 327, pp. 688–702, 2020.
[21]. Cui, G. H., Guo, H. D., Li, H., Zhai, Y., Gong, Z. B., Wu, J., Liu, J. S., Dong, Y. R., Hou, S. X., and Liu, J. R., “RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease, ” Immunity & Ageing, vol. 16, p. 10, 2019.
[22]. Ding, M., Shen, Y., Wang, P., Xie, Z., Xu, S., Zhu, Z., Wang, Y., Lyu, Y., Wang, D., Xu, L., Bi, J., and Yang, H., “Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer's disease, ” Neurochemical Research, vol. 43, no. 11, pp. 2165–2177, 2018.
[23]. Liu, S., Fan, M., Xu, J. X., Yang, L. J., Qi, C. C., Xia, Q. R., and Ge, J. F., “Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology, ” Journal of Neuroinflammation, vol. 19, no. 1, p. 35, 2022.
[24]. Yuyama, K., Sun, H., Sakai, S., Mitsutake, S., Okada, M., Tahara, H., Furukawa, J., Fujitani, N., Shinohara, Y., and Igarashi, Y., “Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice, ” The Journal of Biological Chemistry, vol. 289, no. 35, pp. 24488–24498, 2014.
Cite this article
Zhang,S. (2025). Research Progress of Exosomes in Alzheimer's Disease. Theoretical and Natural Science,126,167-173.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, P., Quan, W., Wang, Z., Liu, Y., Cai, H., Chen, Y., ... and Zhou, Y., “Early-stage differentiation between Alzheimer’s disease and frontotemporal lobe degeneration: Clinical, neuropsychology, and neuroimaging features, ” Frontiers in Aging Neuroscience, vol. 14, 2022, Art. no. 981451.
[2]. Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., Li, Y., Li, Y., Zhu, M., Jiao, H., Song, Y., Shi, Y., Zhang, H., Gong, M., Wei, C., Tang, Y., Fang, B., Guo, D., Wang, F., Zhou, A., et al., “Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study, ” The Lancet Public Health, vol. 5, no. 12, pp. e661–e671, 2020.
[3]. Liu, Q., Tan, Y., Qu, T., Zhang, J., Duan, X., Xu, H., Mu, Y., Ma, H., and Wang, F., “Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro, ” Life Sciences, vol. 254, 2020, Art. no. 117772.
[4]. Caruso Bavisotto, C., Scalia, F., Marino Gammazza, A., Carlisi, D., Bucchieri, F., Conway de Macario, E., Macario, A. J. L., Cappello, F., and Campanella, C., “Extracellular vesicle-mediated cell–cell communication in the nervous system: Focus on neurological diseases, ” International Journal of Molecular Sciences, vol. 20, no. 2, p. 434, 2019.
[5]. Livne-Bar, I., Lam, S., Chan, D., Guo, X., Askar, I., Nahirnyj, A., Flanagan, J. G., and Sivak, J. M., “Pharmacologic inhibition of reactive gliosis blocks TNF-α-mediated neuronal apoptosis, ” Cell Death & Disease, vol. 7, no. 9, p. e2386, 2016.
[6]. Antonucci, F., Turola, E., Riganti, L., Caleo, M., Gabrielli, M., Perrotta, C., Novellino, L., Clementi, E., Giussani, P., Viani, P., Matteoli, M., and Verderio, C., “Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism, ” The EMBO Journal, vol. 31, no. 5, pp. 1231–1240, 2012.
[7]. Sardar Sinha, M., Ansell-Schultz, A., Civitelli, L., Hildesjö, C., Larsson, M., Lannfelt, L., Ingelsson, M., and Hallbeck, M., “Alzheimer's disease pathology propagation by exosomes containing toxic amyloid-beta oligomers, ” Acta Neuropathologica, vol. 136, no. 1, pp. 41–56, 2018.
[8]. Ruan, Z., Pathak, D., Venkatesan Kalavai, S., Yoshii-Kitahara, A., Muraoka, S., Bhatt, N., Takamatsu-Yukawa, K., Hu, J., Wang, Y., Hersh, S., Ericsson, M., Gorantla, S., Gendelman, H. E., Kayed, R., Ikezu, S., Luebke, J. I., and Ikezu, T., “Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons, ” Brain, vol. 144, no. 1, pp. 288–309, 2021.
[9]. Craft, J. M., Watterson, D. M., and Van Eldik, L. J., “Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration, ” Glia, vol. 53, no. 5, pp. 484–490, 2006.
[10]. Manczak, M., Anekonda, T. S., Henson, E., Park, B. S., Quinn, J., and Reddy, P. H., “Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression, ” Human Molecular Genetics, vol. 15, no. 9, pp. 1437–1449, 2006.
[11]. Pei, J. Y., Liu, J. L., Liu, B., Dong, X. H., Cong, S. Y., Ren, X. M., and Zhou, Y. J., “Review of animal models of Alzheimer's disease applied in traditional Chinese medicine research, ” Chinese Journal of Comparative Medicine, pp. 1–14.
[12]. Mao, C. Y., Fang, R. H., and Cong, H., “Progress in the pathogenesis, diagnosis and treatment of Alzheimer’s disease, ” Jiangsu Medical Journal, vol. 51, no. 3, pp. 300–304, 2025.
[13]. Li, Y. X., Wang, Y. R., Liao, J. W., and Mou, L. W., “The role of exosomes in the pathogenesis, diagnosis and treatment of Alzheimer's disease, ” Life Sciences, vol. 37, no. 2, pp. 196–205, 2025.
[14]. Rajendran, L., Honsho, M., Zahn, T. R., Keller, P., Geiger, K. D., Verkade, P., and Simons, K., “Alzheimer’s disease beta-amyloid peptide is released in association with exosomes, ” Proceedings of the National Academy of Sciences of the United States of America, vol. 103, no. 30, pp. 11172–11177, 2006.
[15]. Zheng, T., Pu, J., Chen, Y., Mao, Y., Guo, Z., Pan, H., Zhang, L., Zhang, H., Sun, B., and Zhang, B., “Plasma exosomes spread and cluster around beta-amyloid plaques in an animal model of Alzheimer's disease, ” Frontiers in Aging Neuroscience, vol. 9, p. 12, 2017.
[16]. Saman, S., Kim, W., Raya, M., Visnick, Y., Miro, S., Saman, S., Jackson, B., McKee, A. C., Alvarez, V. E., Lee, N. C., and Hall, G. F., “Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease, ” The Journal of Biological Chemistry, vol. 287, no. 6, pp. 3842–3849, 2012.
[17]. Wang, Y., Balaji, V., Kaniyappan, S., Krüger, L., Irsen, S., Tepper, K., Chandupatla, R., Maetzler, W., Schneider, A., Mandelkow, E., and Mandelkow, E. M., “The release and trans-synaptic transmission of Tau via exosomes, ” Molecular Neurodegeneration, vol. 12, no. 1, p. 5, 2017.
[18]. Asai, H., Ikezu, S., Tsunoda, S., Medalla, M., Luebke, J., Haydar, T., Wolozin, B., Butovsky, O., Kügler, S., and Ikezu, T., “Depletion of microglia and inhibition of exosome synthesis halt tau propagation, ” Nature Neuroscience, vol. 18, no. 11, pp. 1584–1593, 2015.
[19]. Lee, M., Ban, J. J., Yang, S., Im, W., and Kim, M., “The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease, ” Brain Research, vol. 1691, pp. 87–93, 2018.
[20]. Ma, X., Huang, M., Zheng, M., Dai, C., Song, Q., Zhang, Q., Li, Q., Gu, X., Chen, H., Jiang, G., Yu, Y., Liu, X., Li, S., Wang, G., Chen, H., Lu, L., and Gao, X., “ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease, ” Journal of Controlled Release, vol. 327, pp. 688–702, 2020.
[21]. Cui, G. H., Guo, H. D., Li, H., Zhai, Y., Gong, Z. B., Wu, J., Liu, J. S., Dong, Y. R., Hou, S. X., and Liu, J. R., “RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease, ” Immunity & Ageing, vol. 16, p. 10, 2019.
[22]. Ding, M., Shen, Y., Wang, P., Xie, Z., Xu, S., Zhu, Z., Wang, Y., Lyu, Y., Wang, D., Xu, L., Bi, J., and Yang, H., “Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer's disease, ” Neurochemical Research, vol. 43, no. 11, pp. 2165–2177, 2018.
[23]. Liu, S., Fan, M., Xu, J. X., Yang, L. J., Qi, C. C., Xia, Q. R., and Ge, J. F., “Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology, ” Journal of Neuroinflammation, vol. 19, no. 1, p. 35, 2022.
[24]. Yuyama, K., Sun, H., Sakai, S., Mitsutake, S., Okada, M., Tahara, H., Furukawa, J., Fujitani, N., Shinohara, Y., and Igarashi, Y., “Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice, ” The Journal of Biological Chemistry, vol. 289, no. 35, pp. 24488–24498, 2014.









