1. Introduction
Mushroom poisoning is a persistent public health problem, especially in regions where wild mushroom foraging is common. Globally, the World Health Organisation (WHO) estimates that over 10,000 cases of mushroom poisoning are reported each year, with several hundred resulting in fatalities. Notably, China documented more than 1,400 cases and 44 deaths in 2022, while Europe and North America continue to see periodic outbreaks, often linked to seasonal foraging activities and the misidentification of toxic species as edible varieties. Cyclopeptides—from Amanita species—are responsible for most fatal cases due to their potent hepatotoxic effects. Orellanine, found in Cortinarius mushrooms, presents a unique challenge with its delayed onset of renal toxicity, which often leads to misdiagnosis and late intervention. Hydrazine poisoning, especially from false morels, is characterized by severe neurological and hepatic complications and remains a concern in areas where these mushrooms are part of traditional diets.
Despite advances in analytical chemistry and toxicology, rapid and accurate identification of mushroom toxins in clinical settings is still inadequate. Current management is supportive, as specific antidotes for most mushroom toxins are unavailable. Moreover, public awareness of the risks associated with wild mushroom consumption remains insufficient, particularly among amateur foragers. Existing research have summarized clinical manifestations and general management, but a comprehensive synthesis focusing on recent advances in molecular mechanisms, diagnostic strategies, and prevention is still lacking. This review aims to fill these gaps by systematically summarizing the biochemical pathways, clinical presentations, and treatment options for major mushroom toxins, and by emphasizing the urgent need for improved diagnostic tools, specific therapies, and targeted public education to mitigate the global burden of mushroom poisoning.
2. Cyclopeptides
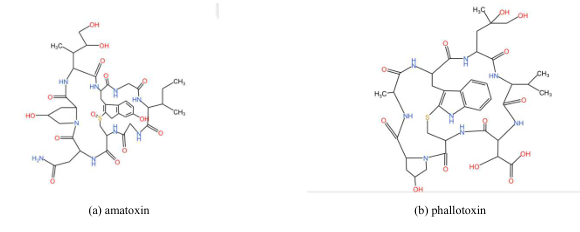
Here we can set a diagram to show the structure and features of these two molecules [2- 4].
|
α-amanitin |
Phallacidin |
|
|
Ring skeleton |
8 amino acid that from a closed ring structure which is a bicyclist octapeptide |
7 amino acid and a hydro proline which from a bicyclist heptapeptide |
|
Cross linking method |
Rigid double -ring system via two thioether (the bicyclist structure makes it highly rigid that increase its stability under elevated temperature and protease hydrolysis) |
Double ring formed by one thioether and an ether bond (the presence of ether bond will increase the flexibility of the molecule) |
|
Amino acid composition |
Hydroxyisoleucine (enhanced hydrophilicity) Tryptophan (take part in target binding) |
Hydroxyproline (increase molecularpolarity) Cysteine (provides self-hydro groups) |
2.1. Molecular targets and pathways of cyclopeptides
The structure of the α-amanitin is shown in figure 1. And table 1 shows differences in structure between the two cyclopeptides toxin. Mechanism of toxicity α-amanitin will be absorbed rapidly by the stomach and enter the liver through the portal vein, which will lead to hepatotoxicity [5]. (It is worth mentioning that α-amanitin has been shown to work synergistically with TNF-alpha, which is a tumor necrosis factor that may be the reason for liver failure [6]) inhibit causes and cause a decline in content of mRNA as it will implant the active center of RNAPII then bind with bridge helix and trigger loop to block the extension of mRNA chain [7]. Hence, there will be inadequate protein synthesis and cell death because of the stop of synthesis of mRNA caused by the inactivation of RNAPII [3].
An experiment conducted by Ping Zhou et al has also described the liver failure after injection of α-amanitin [8]. The liver enzymes its peak after 36 hours and decline sharply. What is more, MRI has shown abnormal liver signals, and ultrasound results have shown an increase in hepatic parenchyma echo and decreased blood perfusion [8]. Liver biopsy revealed severe steatosis of liver cells after 36 hours, and mass necrosis occurred after 49 hours [8]. Ping Zhou et al also refer that α-amanitin will cause cerebral oedema since brain MRI found abnormal signal in the frontal and temporal [8].
α-amanitin has been proved to activate the p53 mitochondrial apoptosis pathway through IGFBP1 [9]. Donna L. et al have used a test to exhibit the mechanism. As α-amanitin inhibits RNAPII and blocks IGFBP1 (which depend on transcriptional activation of p53), that will cause the accumulation of p53 mitochondria and binding to BAK (a pro-apoptotic protein), hence activating the apoptosis process [9]. In the mouse liver, α-amanitin induced p53 mitochondria localization, BAK oligomerisation, which comes with extensive hepatocyte apoptosis. It is worth mentioning that the p53 / BAK pathway is the key to the mechanism of hepatotoxicity since the mice with p53 or BAK deficit have been observed to have no significant biomarker of apoptotic after the α-amanitin treatment. This indicates a significant reduction in liver failure during the experiment [9].
In the study of Willemien F. J. Hof et al, the concentration-dependent and time dependent toxic effects on multiple human hematopoietic progenitor cell lines and primary human CD34+ stem cells of α-amanitin have been demonstrated for the first time [10].
2.2. Toxicokinetics and pharmacokinetics
Research have shown that that α-amanitin does not undergo significant monism effect. While its excretion pathways and clearance mechanisms significantly affect its recovery rate and its effects on the liver. Intravenous (IV) administration in rats resulted in a total recovery of 68.9%, with the majority of the administered α-amanitin excreted within 8 hours, suggesting the involvement of specific renal transporters in its clearance [11]. In contrast, the oral (PO) group had a significantly lower recovery rate of only 23.5%, with most excretion occurring via faeces [11]. Due to the restricted collection period characteristic of the semi-mass balance study design [11], the recovery may have been lower than anticipated. The results showed that no notable metabolites were found in rat plasma in in vivo, and there was no evidence of reactive metabolites like glutathione (GSH) adducts [11]. However, trace amounts of glucuronide metabolites were identified in liver microsomes in vitro, at levels slightly higher than expected (14 mL/min/kg) [11].
Research has shown that α-amanitin is primarily eliminated through bile, with an enterohepatic circulation potentially prolonging hepatotoxic effect [12]. Studies in mice have demonstrated that low-level exposure to α-amanitin leads to renal lesions, whereas high-level exposure typically results in death due to liver failure or hypoglycemia before renal lesions manifest [12].
2.3. Clinical symptoms and treatment
The oral intake of amatoxin peptides is extremely lethal to humans, and its typical clinical course presents a biphasic feature of ‘symptom relief - organ failure.’ Effective treatment requires a three - dimensional clearance strategy that simultaneously implements gastrointestinal decontamination, interception of circulation of circulating toxins, and enhancement of renal excretion. The LD50 of α-amanitin in humans is estimated to be 0.1 mg/kg when administered per os (by mouth) [12]. α-amanitin poisoning leads to the so called” phalloid syndrome” [12]. Nausea, vomiting, diarrhoea, and stomach pain typically occur in the first stage and continue for up to 24 hours (with the initial 6- 8 hours often being an asymptomatic latent phase) [12, 13]. This is followed by a remission period lasting for 24 to 36 hours [12]. In this period, AST and ALT levels progressively rise, indicating the onset of liver damage [12]. The third phase, typically occurring 36 to 38 hours post-ingestion, represents a seeming recovery stage—gastrointestinal symptoms may subside temporarily, yet coagulopathy worsens, and liver function tests become significantly abnormal [13]. The final stage, occurring 4- 7 days after ingestion, is distinguished by renal and hepatic impairment that can lead to hepatic encephalopathy, coma, and death [12].
Gastric decontamination—typically involving both aspiration and lavage alongside gastric tube insertion—can assist in minimizing toxin reabsorption [13, 14]. Given that amatoxins undergo enterohepatic circulation, multiple doses of activated charcoal are advised to bind biliary toxins within the intestines and inhibit reabsorption [13]. Amatoxins may be present in urine for up to four days following ingestion [13], highlighting the role of enhanced renal clearance in toxin elimination [13]. Recommended interventions include intravenous fluid administration and diuresis, aiming to maintain urine output between 100 to 200 mL per hour for 4 to 5 days to promote renal excretion of amatoxins [13].
3. Orellanine and related toxins
3.1. Overview of orellanine and related toxins
Orellanine is a naturally occurring bipyridine toxin, discovered from certain species within the Cortinarius genus, notably Cortinarius orellanus and Cortinarius rubellus [15]. These species are often misidentified as edible due to their morphological similarities, contributing to unintended toxic exposures. What distinguishes orellanine from other mycotoxins is its pronounced nephrotoxic effect, with the kidney being the principal organ. In severe cases, it can progress to acute renal failure, sometimes necessitating long-term renal replacement therapy or even transplantation [16].
3.2. Chemical structure and toxic mechanism of orellanine
Structurally, orellanine is defined by a bipyridine core substituted with four hydroxyl groups and two N-oxide functionalities, forming a 3,3,4,4-tetrahydroxy-2,2-bipyridine1,1-dioxide framework [17]. This configuration facilitates redox cycling within biological systems, generating reactive oxygen species (ROS). These ROS play a vital role in the induction of oxidative stress, resulting in renal tubular epithelial cell apoptosis and necrosis [18]. Additionally, orrellanine disrupts mitochondrial respiration and interferes with metal ion regulation, further exacerbating renal cellular injury [19].
3.3. Clinical features and diagnosis of orellanine poisoning
Clinically, orellanine poisoning is characterized by a delayed onset of symptoms. Most patients remain asymptomatic for 3 to 20 days post-ingestion, a latency that frequently complicates early diagnosis [20]. The initial presentation is nonspecific, including nausea, vomiting, fatigue, and thirst. As renal damage progresses, patients may exhibit polyuria, hematuria, or eventually oliguria and anuria, reflective of declining kidney function [21]. Because of this delay, clinicians often fail to associate renal failure with mushroom ingestion, delaying critical therapeutic interventions [16].
3.4. Treatment strategies and prognosis
Currently, there is no specific antidote for orellanine. Management is supportive. Prompt initiation of intravenous fluid resuscitation and renal replacement therapy, particularly hemodialysis, may mitigate progression to end-stage renal disease [15]. In cases of irreversible damage, renal transplantation remains the only definitive treatment. The overall prognosis is closely tied to both the dose of toxin ingested and the timeliness of clinical response [17].
3.5. Epidemiology and historical cases
The first well-documented outbreak of orellanine poisoning was reported in Poland during the 1950s, when several individuals developed renal failure following consumption of Cortinarius mushrooms mistakenly identified as edible species [18]. Subsequent cases have been documented across Europe and North America, underscoring the widespread distribution of toxic Cortinarius species and the global public health concern they pose [19].
3.6. Other related toxins and analytical challenges
Beyond orellanine itself, structurally related compounds such as orelline and cortinarins have also been identified in Cortinarius species. While their precise toxicological roles remain under investigation, their co-occurrence is believed to contribute to the complex toxic profiles of these mushrooms [20]. Their presence also adds a layer of difficulty to both clinical management and analytical identification during toxicological assessment [21].
3.7. Importance of public awareness and risk prevention
Given the visual similarity of toxic Cortinarius species to non-toxic, edible varieties, public education and accurate identification are of utmost importance. Foragers should avoid wild mushroom consumption unless professionally trained or guided by experts, as misidentification can lead to life-threatening consequences [15].
4. Hydrazines
4.1. Chemical origin and structure
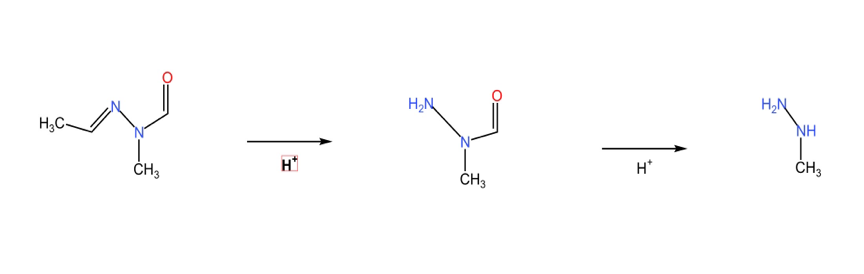
As illustrated in Figure 2, buck flower undergoes hydrolysis to yield N-methyl-N-hydrazine, which is subsequently transformed into MMH. This oily chemical has been discovered since 1885 from a fungus called Gyromitra, commonly known as the false morel [22]. According to in-depth research, this substance is a mixture of a variety of non-toxic organic acids, including valeraldehydeN-methyl-N-formylhydrazide, 3-methylbutyraldehyde-N-methyl-N-formylhydrazide, and hexanal-N-methyl-N-formylhydrazide, all of which can produce MMH by hydrolysis [23]. The chemical formula of N-methyl-N-hydrazine is C4H8N2O, and the chemical name is acetaldehyde-N-methyl-N-formylhydrazine, which has the characteristics of volatility, heat sensitivity, and high-water solubility [24, 25].
4.2. Mechanisms of toxicity
Its toxic mechanism is classified as a mycotoxin that inhibits GABA, which the human body metabolises and produces MMH. GABA is an inhibitory neurotransmitter in the central nervous system. Inhibition of this function can lead to the loss of control of neuronal activity. Its excitability will be enhanced, which may lead to abnormal synchronized discharge, resulting in epilepsy [26].
In the field of neurotoxins, MMH binds to pyridoxine-dependent coenzymes and thus acts as an inhibitor [27], inhibiting glutamate decarboxylase activity, reducing γ-aminobutyric acid synthesis, and finally leading to neurological symptoms like epilepsy. Secondly, MMH can also cause methemoglobinemia, which is a problem in the field of hemotoxicity. Third, within the liver, free radicals produced by MMH may cause hepatocyte lysis. The free radicals attack liver cell membranes and DNA, leading to the inability to transcribe DNA, resulting in liver necrosis and even cancer [22, 23]. Therefore, deer flower toxin is also considered a carcinogen.
4.3. Industrial applications and environmental exposure
In terms of aetiology, this toxin has a wide range of applications, such as being used as rocket fuel during World War II in Germany [28], or as an intermediate for plastic foaming agents or agricultural herbicides in chemical production. It can also serve as a deoxidizer for iron to prevent corrosion of boiler pipelines [29].
4.4. Clinical toxicology and management
According to studies, the fatality rate of the toxin is about 10% [22]. The individual response to the toxin varies, from mild gastrointestinal discomfort to direct death. In the literature, methylhydrazine tolerance is 4.8 to 8 mg/kg in adults and 1.6 to 4.8 mg/kg in children [27].
Treatment includes the injection of vitamin B6 to control epilepsy, as vitamin B6 acts as a coenzyme for glutamic acid decarboxylase, partially restoring GABA synthesis. Alternatively, benzodiazepines can be used for sedation to inhibit neuronal hyperexcitability and enhance GABA receptor activity. Early intervention methods include vomiting or gastric lavage to clear unabsorbed toxins.
Due to its similarity to morels, the mushroom is often mistaken for edible species, especially in the northern hemisphere, leading to numerous poisoning deaths. The incubation period is long, 5–12 hours [27]. Initial symptoms include nausea, vomiting, diarrhea, liver dysfunction, impaired consciousness, coma, and eventually death. The first recorded case of deer flower poisoning was in 1782. The small number of reported cases may be due to symptom misdiagnosis, low mortality rate, or degradation of the toxins by traditional cooking methods [30].
Geographical distribution and food customs, particularly in Eastern Europe, where heat treatment is believed to remove toxicity, contribute to the risk of poisoning.
4.5. Occupational and accidental exposure incidents
In the industrial sector, fatal examples include worker deaths after prolonged exposure to this toxin for six months [31]. Common exposure pathways include inhalation from rocket fuel leaks, accidental ingestion of liquid hydrazine by crew members, and accidental skin contact during gilding or weeding, all of which can lead to poisoning [32].
5. Discussion
The clinical management of mushroom poisoning remains challenging due to several factors, including the delayed onset of symptoms associated with orellanine and the nonspecific initial presentations of cyclopeptide and hydrazine toxicities. These diagnostic hurdles highlight the critical need for rapid, sensitive, and specific analytical methods to identify toxins promptly, enabling early intervention. Although current treatments primarily rely on supportive care, the lack of specific antidotes continues to limit therapeutic outcomes, especially in severe cases. The development of novel antidotes and protective agents, guided by an improved understanding of the biochemical mechanisms underlying mushroom toxicity, is essential. Furthermore, targeted public health initiatives focusing on education and awareness could significantly mitigate the incidence of mushroom poisoning by reducing accidental ingestions, particularly in high-risk areas and populations.
6. Conclusion
Mushroom poisoning continues to pose a significant threat to global public health, with cyclopeptides, orellanine, and hydrazines being responsible for the most severe cases of toxicity. Cyclopeptides such as amatoxins induce rapid and potentially fatal liver failure, while orellanine leads to delayed-onset, often irreversible kidney injury. Hydrazinecontaining mushrooms primarily affect the nervous system and can cause additional hepatic and haematological complications. Despite advances in understanding the molecular mechanisms of these toxins, clinical management remains supportive due to the absence of specific antidotes and the challenges of rapid, accurate diagnosis. Improving analytical detection methods, developing targeted therapeutic strategies, and strengthening public education are urgently needed to reduce the incidence and severity of mushroom poisoning. Continued interdisciplinary research and effective public health initiatives will be essential to mitigate the global burden of this persistent toxicological threat. Furthermore, the integration of molecular diagnostics, public health policy, and education will be vital in reducing the incidence and severity of mushroom poisoning on a global scale.
Acknowledgements
These authors contributed equally to this work.
References
[1]. Jonathan D Walton, Heather E Hallen-Adams, and Hong Luo. Ribosomal biosynthesis of the cyclic peptide toxins of amanita mushrooms. Peptide Science, 94(5): 659– 664, 2010.
[2]. Theodor Wieland, Heinz Faulstich, and Luigi Fiume. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous amanita mushroom. CRC critical reviews in biochemistry, 5(3): 185–260, 1978.
[3]. Irene Gouvinhas, Jani Silva, Maria José Alves, and Juliana Garcia. The most dreadful mushroom toxins: a review of their toxicological mechanisms, chemical structural characteristics, and treatment. EXCLI journal, 23: 833, 2024.
[4]. Mustafa Oğuz Tuğcan and Ayça Açıkalın Akpınar. Mushroom poisoning: An updated review. Turkish Journal of Emergency Medicine, 25(1): 10–16, 2025.
[5]. Patrick Poucheret, Françoise Fons, Jean Christophe Doré, Didier Michelot, and Sylvie Rapior. Amatoxin poisoning treatment decision-making: pharmacotherapeutic clinical strategy assessment using multidimensional multivariate statistic analysis. Toxicon, 55(7): 1338–1345, 2010.
[6]. Marcel Leist, Florian Gantner, Heike Naumann, Horst Bluethmann, Kathrin Vogt, Regina Brigelius-Flohe, Pierluigi Nicotera, Hans-Dieter Volk, and Albrecht Wendel. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. 1997.
[7]. Chong Zheng, Shaofang Lv, Jianfang Ye, Lu Zou, Kai Zhu, Haichang Li, Yongxi Dong, and Lei Li. Metabolomic insights into the mechanisms of ganoderic acid: protection against α-amanitin-induced liver injury. Metabolites, 13(11): 1164, 2023.
[8]. Ping Zhou, Jie Xia, Gang Guo, Zi-Xing Huang, Qiang Lu, Li Li, Hong-Xia Li, YuJun Shi, and Hong Bu. A macaca mulatta model of fulminant hepatic failure. World Journal of Gastroenterology: WJG, 18(5): 435, 2012.
[9]. JI-Ju Leu and Donna L George. Hepatic igfbp1 is a prosurvival factor that binds to bak, protects the liver from apoptosis, and antagonizes the proapoptotic actions of p53 at mitochondria. Genes & development, 21(23): 3095–3109, 2007.
[10]. Willemien FJ Hof, Miranda Visser, Joyce J de Jong, Marian N Rajasekar, Jan Jacob Schuringa, Inge AM de Graaf, Daan J Touw, and Bart GJ Dekkers. Unraveling hematotoxicity of α-amanitin in cultured hematopoietic cells. Toxins, 16(1): 61, 2024.
[11]. Jiyu Lee, Byeong Ill Lee, Jangmi Choi, Yuri Park, Seo-Jin Park, Minjae Park, JeongHyeon Lim, Sangsoo Hwang, Jeong-Min Lee, and Young G Shin. Investigation of in vitro and in vivo metabolism of α-amanitin in rats using liquid chromatographyquadrupole time-of-flight mass spectrometric method. Molecules, 27(23): 8612, 2022.
[12]. Estelle Flament, Jérôme Guitton, Jean-Michel Gaulier, and Yvan Gaillard. Human poisoning from poisonous higher fungi: Focus on analytical toxicology and case reports in forensic toxicology. Pharmaceuticals, 13(12): 454, 2020.
[13]. Tahrima Kayes and Vincent Ho. Amanita phalloides-associated liver failure: Molecular mechanisms and management. International Journal of Molecular Sciences, 25(23): 13028, 2024.
[14]. Maximiliano Rovegno, Magdalena Vera, Alex Ruiz, and Carlos Benítez. Current concepts in acute liver failure. Annals of hepatology, 18(4): 543–552, 2019.
[15]. JM Richard and et al. Nephrotoxicity of orellanine, a toxin from the mushroom cortinarius orellanus. Archives of Toxicology, 62(2): 110–113, 1988.
[16]. RG Hendrickson. Orellanine mushroom toxicity, 2023. Medscape Reference.
[17]. S Horn and et al. End-stage renal failure from mushroom poisoning with cortinarius orellanus: Report of four cases and review of the literature. American Journal of Kidney Diseases, 30(2): 256–260, 1997.
[18]. BS Judge and et al. Ingestion of a newly described north american mushroom species resulting in chronic renal failure: Cortinarius orellanosus. Clinical Toxicology, 48(6): 545–549, 2010.
[19]. RJ Dinis-Oliveira and et al. Human and experimental toxicology of orellanine. Human & Experimental Toxicology, 34(9): 845–864, 2015.
[20]. MJ Lyons and et al. Orellanine: From fungal origin to a potential future cancer treatment. Journal of Natural Products, 86(6): 1620–1631, 2023.
[21]. X Shao and et al. A novel orellanine containing mushroom cortinarius armillatus. Toxicon, 118: 1–5, 2016.
[22]. D. Michelot and B. Toth. Poisoning by gyromitra esculenta—a review. Journal of Applied Toxicology, 11(4): 235–243, 1991.
[23]. J. Patocka, R. Pita, and K. Kuca. Gyromitrin, mushroom toxin of gyromitra spp. Military Medical Science Letters, 81(2): 61–67, 2012.
[24]. H. Pyysalo. Some new toxic compounds in false morels, gyromitra esculenta. Naturwissenschaften, 62(8): 395, 1975.
[25]. H. Pyysalo. Tests for gyromitrin, a poisonous compound in false morel gyromitra esculenta. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 160(3): 325– 330, 1976.
[26]. J. White, S.A. Weinstein, L. De Haro, R. Bédry, A. Schaper, B.H. Rumack, and T. Zilker. Mushroom poisoning: A proposed new clinical classification. Toxicon, 157: 53–65, 2019.
[27]. A.M. Leathem and T.J. Dorran. Poisoning due to raw gyromitra esculenta (false morels) west of the rockies. Canadian Journal of Emergency Medicine, 9(2): 127– 130, 2007.
[28]. L.R. Cook, R.E. Glenn, and G.E. Podolak. Monitoring and analysis of personnel exposures to hydrazines at a rocket propellant plant. American Industrial Hygiene Association Journal, 40(1): 69–74, 1979.
[29]. P.S. Spencer and G.E. Kisby. Role of hydrazine-related chemicals in cancer and neurodegenerative disease. Chemical Research in Toxicology, 34(9): 1953–1969, 2021.
[30]. S. Franke, U. Freimuth, and P.H. List. Über die giftigkeit der frühjahrslorchel gyromitra (helvella) esculenta fr. 14. pilzinhaltsstoffe. Archives of Toxicology, 22(5): 293–332, 1967.
[31]. E. Sotaniemi, J. Hirvonen, H. Isomäki, J. Takkunen, and J. Kaila. Hydrazine toxicity in the human. report of a fatal case. Annals of Clinical Research, 3(1): 30–33, 1971.
[32]. K. Wrangsjö and A. Mårtensson. Hydrazine contact dermatitis from gold plating. Contact Dermatitis, 15(4): 244–245, 1986.
Cite this article
Chen,Z.;Mao,Y.;Wang,K. (2025). The Mechanism and Application of Mushroom Toxicity. Theoretical and Natural Science,117,117-125.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jonathan D Walton, Heather E Hallen-Adams, and Hong Luo. Ribosomal biosynthesis of the cyclic peptide toxins of amanita mushrooms. Peptide Science, 94(5): 659– 664, 2010.
[2]. Theodor Wieland, Heinz Faulstich, and Luigi Fiume. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous amanita mushroom. CRC critical reviews in biochemistry, 5(3): 185–260, 1978.
[3]. Irene Gouvinhas, Jani Silva, Maria José Alves, and Juliana Garcia. The most dreadful mushroom toxins: a review of their toxicological mechanisms, chemical structural characteristics, and treatment. EXCLI journal, 23: 833, 2024.
[4]. Mustafa Oğuz Tuğcan and Ayça Açıkalın Akpınar. Mushroom poisoning: An updated review. Turkish Journal of Emergency Medicine, 25(1): 10–16, 2025.
[5]. Patrick Poucheret, Françoise Fons, Jean Christophe Doré, Didier Michelot, and Sylvie Rapior. Amatoxin poisoning treatment decision-making: pharmacotherapeutic clinical strategy assessment using multidimensional multivariate statistic analysis. Toxicon, 55(7): 1338–1345, 2010.
[6]. Marcel Leist, Florian Gantner, Heike Naumann, Horst Bluethmann, Kathrin Vogt, Regina Brigelius-Flohe, Pierluigi Nicotera, Hans-Dieter Volk, and Albrecht Wendel. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. 1997.
[7]. Chong Zheng, Shaofang Lv, Jianfang Ye, Lu Zou, Kai Zhu, Haichang Li, Yongxi Dong, and Lei Li. Metabolomic insights into the mechanisms of ganoderic acid: protection against α-amanitin-induced liver injury. Metabolites, 13(11): 1164, 2023.
[8]. Ping Zhou, Jie Xia, Gang Guo, Zi-Xing Huang, Qiang Lu, Li Li, Hong-Xia Li, YuJun Shi, and Hong Bu. A macaca mulatta model of fulminant hepatic failure. World Journal of Gastroenterology: WJG, 18(5): 435, 2012.
[9]. JI-Ju Leu and Donna L George. Hepatic igfbp1 is a prosurvival factor that binds to bak, protects the liver from apoptosis, and antagonizes the proapoptotic actions of p53 at mitochondria. Genes & development, 21(23): 3095–3109, 2007.
[10]. Willemien FJ Hof, Miranda Visser, Joyce J de Jong, Marian N Rajasekar, Jan Jacob Schuringa, Inge AM de Graaf, Daan J Touw, and Bart GJ Dekkers. Unraveling hematotoxicity of α-amanitin in cultured hematopoietic cells. Toxins, 16(1): 61, 2024.
[11]. Jiyu Lee, Byeong Ill Lee, Jangmi Choi, Yuri Park, Seo-Jin Park, Minjae Park, JeongHyeon Lim, Sangsoo Hwang, Jeong-Min Lee, and Young G Shin. Investigation of in vitro and in vivo metabolism of α-amanitin in rats using liquid chromatographyquadrupole time-of-flight mass spectrometric method. Molecules, 27(23): 8612, 2022.
[12]. Estelle Flament, Jérôme Guitton, Jean-Michel Gaulier, and Yvan Gaillard. Human poisoning from poisonous higher fungi: Focus on analytical toxicology and case reports in forensic toxicology. Pharmaceuticals, 13(12): 454, 2020.
[13]. Tahrima Kayes and Vincent Ho. Amanita phalloides-associated liver failure: Molecular mechanisms and management. International Journal of Molecular Sciences, 25(23): 13028, 2024.
[14]. Maximiliano Rovegno, Magdalena Vera, Alex Ruiz, and Carlos Benítez. Current concepts in acute liver failure. Annals of hepatology, 18(4): 543–552, 2019.
[15]. JM Richard and et al. Nephrotoxicity of orellanine, a toxin from the mushroom cortinarius orellanus. Archives of Toxicology, 62(2): 110–113, 1988.
[16]. RG Hendrickson. Orellanine mushroom toxicity, 2023. Medscape Reference.
[17]. S Horn and et al. End-stage renal failure from mushroom poisoning with cortinarius orellanus: Report of four cases and review of the literature. American Journal of Kidney Diseases, 30(2): 256–260, 1997.
[18]. BS Judge and et al. Ingestion of a newly described north american mushroom species resulting in chronic renal failure: Cortinarius orellanosus. Clinical Toxicology, 48(6): 545–549, 2010.
[19]. RJ Dinis-Oliveira and et al. Human and experimental toxicology of orellanine. Human & Experimental Toxicology, 34(9): 845–864, 2015.
[20]. MJ Lyons and et al. Orellanine: From fungal origin to a potential future cancer treatment. Journal of Natural Products, 86(6): 1620–1631, 2023.
[21]. X Shao and et al. A novel orellanine containing mushroom cortinarius armillatus. Toxicon, 118: 1–5, 2016.
[22]. D. Michelot and B. Toth. Poisoning by gyromitra esculenta—a review. Journal of Applied Toxicology, 11(4): 235–243, 1991.
[23]. J. Patocka, R. Pita, and K. Kuca. Gyromitrin, mushroom toxin of gyromitra spp. Military Medical Science Letters, 81(2): 61–67, 2012.
[24]. H. Pyysalo. Some new toxic compounds in false morels, gyromitra esculenta. Naturwissenschaften, 62(8): 395, 1975.
[25]. H. Pyysalo. Tests for gyromitrin, a poisonous compound in false morel gyromitra esculenta. Zeitschrift für Lebensmittel-Untersuchung und -Forschung, 160(3): 325– 330, 1976.
[26]. J. White, S.A. Weinstein, L. De Haro, R. Bédry, A. Schaper, B.H. Rumack, and T. Zilker. Mushroom poisoning: A proposed new clinical classification. Toxicon, 157: 53–65, 2019.
[27]. A.M. Leathem and T.J. Dorran. Poisoning due to raw gyromitra esculenta (false morels) west of the rockies. Canadian Journal of Emergency Medicine, 9(2): 127– 130, 2007.
[28]. L.R. Cook, R.E. Glenn, and G.E. Podolak. Monitoring and analysis of personnel exposures to hydrazines at a rocket propellant plant. American Industrial Hygiene Association Journal, 40(1): 69–74, 1979.
[29]. P.S. Spencer and G.E. Kisby. Role of hydrazine-related chemicals in cancer and neurodegenerative disease. Chemical Research in Toxicology, 34(9): 1953–1969, 2021.
[30]. S. Franke, U. Freimuth, and P.H. List. Über die giftigkeit der frühjahrslorchel gyromitra (helvella) esculenta fr. 14. pilzinhaltsstoffe. Archives of Toxicology, 22(5): 293–332, 1967.
[31]. E. Sotaniemi, J. Hirvonen, H. Isomäki, J. Takkunen, and J. Kaila. Hydrazine toxicity in the human. report of a fatal case. Annals of Clinical Research, 3(1): 30–33, 1971.
[32]. K. Wrangsjö and A. Mårtensson. Hydrazine contact dermatitis from gold plating. Contact Dermatitis, 15(4): 244–245, 1986.









