1. Introduction
Lead (Pb) is a heavy metal that can be found in dust, water, soil, and food that has been cultivated in soil that contains a lot of lead [1]. In the construction business, lead is widely employed because of its special physicochemical qualities. Lead is a persistent metal that is harmful to both people and animals, particularly young ones. According to Kim H.-C. et al. (2015), human exposure to lead persists despite extensive efforts by governments worldwide to minimize lead contamination. The hazards that lead poses to health, shown mostly as injury to the central nervous system and have been identified as a significant public health concern. Numerous studies have demonstrated that physical alterations can result from even modest levels of lead exposure. An example of a dangerous blood lead level is 10 micrograms per deciliter (equal to 0.48 micromoles per liter) or more, which can cause hypertension, neurological abnormalities, and other ailments [2].
Following exposure, lead attaches itself to circulating red blood cells and enters the bloodstream as lead ions (pb2+) [3]. It has about a 30-day half-life.
It then spreads throughout the body, making short-term deposits in the kidneys, liver, and brain before spending up to 20 to 30 years in storage in the bones. A risk factor for lead ion poisoning and its consequences is nutritional status. Deficits in iron (Fe2+), zinc (Zn2+), and calcium (Ca2+) thereby increase the absorption of lead ions in the gastrointestinal tract and impact the vulnerability to the neurotoxicity of lead ions, which will be further discussed in the subsequent sections of this chapter. Pregnancy, menopause, lactation, and aging are other physiological states that encourage the release of metals from storage locations.
The laboratory simulates human exposure to lead ions using a variety of animal models, primarily mice, primarily by gavage, intraperitoneal injection, or drinking water administration. Following absorption, lead ions disrupt several organs, including the kidneys, liver, and central nervous system (CNS), particularly the most susceptible systems during development.
2. The influence of maternal lead exposure on the cerebellum
2.1. Methodology
Mice are used because of the great degree of genetic similarity between them and humans. Consequently, the researchers produced male and female wild-type C57BL/6 mice (7 weeks old) in Dankook University's animal facility using DBL (Korea Umson) as their source [4]. To mimic how the human body might react to lead exposure, these mice were used in place of humans in trials. To facilitate mating, the purchased mice were kept in plastic cages in a 1:2 male to female ratio. The temperature was kept between 21 and 23°C, the relative humidity was kept between 40 and 60%, and there was a 12-hour light-dark cycle from 8:00 to 20:00. There was always an abundance of food and water available.
For the control group and the lead exposure groups, three mating cages were set up. The animals exposed to lead were then separated into two groups: Pregnancy (P) and Pregnancy + Lactation (P + L). According to Leasure et al. (2008), the lead exposure groups were given water that included 27 parts per million of lead acetate (PbAc; Sigma-Aldrich, St. Louis, Missouri, USA), while the control group was given untreated water. Following mating, the lead-treated water was consumed by the lead exposure groups one week later. The P group stopped receiving the lead-containing water solution after the child was born, but the P + L group kept getting it until the child reached postnatal day 28 (PND). Every test and analysis were conducted during the fourth week following delivery.
After the behavioral tests, the children of mothers exposed to lead were put to death, and their brains were taken. The mice were put to sleep with halothane (2-bromo-2-chloro-1,1,1-trifluoroethane) from Sigma-Aldrich in St. Louis, Missouri, USA. With the use of forceps, the mice's skulls were carefully removed. The cerebellums that had been harvested were put in 2 mL screw-cap tubes and quickly frozen in liquid nitrogen. To extract the RNA later, the frozen cerebellums were kept in a deep freezer at -80°C.
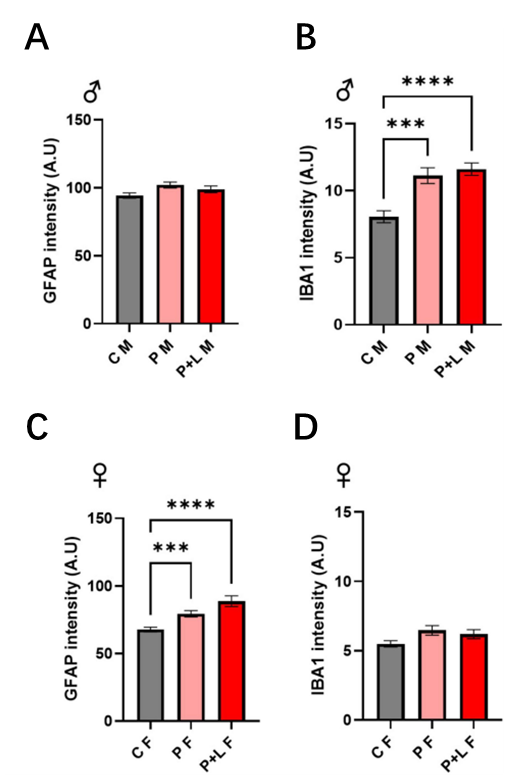
2.2. Result
They investigated if gliosis response might be induced by maternal lead exposure and whether exposure length affected this phenomenon. First, they evaluated the degree of gliosis in the progeny after exposure to lead. Only microglial proliferation was seen in the male progeny. In all groups, GFAP expression was unchanged (Figure 1C, D). . Moreover, Western blot analysis confirmed these modifications. These results imply that lead exposure during both P and PCL periods may cause gliosis in the cerebellum.
3. Occupational lead exposure and amyotrophic lateral sclerosis
The Cox proportional hazards model was used to calculate the hazard ratios (HR) and 95% confidence intervals (CI) for the associations between lead exposure, covariates, and survival [5]. The range of feasible time points was identified from the beginning of symptoms to the survival endpoint (death, tracheostomy, or PAV) or censoring (the final day of survival without tracheostomy or PAV). As a secondary analysis, the relationship between lead exposure and the rate of ALSFRS-R functional decline according to each PGB study assessment record was examined using an unstructured covariance matrix and a mixed-effects linear regression model with random intercepts and slopes.
The clinical features, genetic profiles, and survival patterns of 135 individuals with amyotrophic lateral sclerosis (ALS) are examined in this study. It is shown that patients with spinal onset are more likely to be male. The beginning age and BMI are within normal limits, and the durations of symptoms till diagnosis, first appointment, and questionnaire completion show regular trends. Most patients exhibit distributions in their smoking history, C9orf72 mutation status, and baseline functional scores. According to survival study, a worse prognosis is linked to bulbar onset, greater age at onset, and a shorter diagnostic delay.
Out of 135 individuals with ALS, six (4%) were diagnosed with ALS-FTSD (Table 1). Men accounted for 86 cases (64%) and the majority (n=99,73%) had spinal onset. A mean age (± standard deviation) of 56.1 years (± 12.8) and a body mass index (BMI) of 26.1 (± 5.0) kg/m² were recorded at the onset of symptoms. The median interval between the diagnosis of ALS and the beginning of symptoms was 1.0 years (0.6-2.0, 25th-75th percentile). 0.8 years was the median interval between diagnosis and first consultation (25th-75th percentile, 0.3-2.3), while 0.5 years was the median interval between first consultation and completion of environmental questionnaires (25th-75th percentile, 0.1-1.2). A median of 3.2 years passed between the onset of symptoms and the completion of the questionnaire (25th-75th percentile, 1.9-5.4).
In the 25th to 75th percentile, the median baseline ALSFRS-R score was 37.0 (32.0-41.0). Of the patients, 7 (5%) had the C9orf72 repeat expansion mutation, and the majority (59%), had never smoked.
Throughout the 817 person-year follow-up period, 38 patients (28%) achieved the survival objective. After symptoms started, the median survival period without PAV and tracheostomy was 48.3 months (25th-75th percentile, 30.9-74.1). Bivariate analysis revealed that the only characteristics significantly linked to lower survival were bulbar onset, older age at onset, and shorter diagnostic delay. Despite having higher hazard ratios, the numbers in the C9orf72 mutation and ALS-FTSD condition were not extremely high.
27% of the 135 ALS patients had occupational lead exposure (mostly from low-intensity military duty), which was associated with worse C9orf72 positive and higher frequencies of males and smokers. Of those exposed, 31% met survival objectives throughout follow-up.
Of the 135 ALS patients, 36 (27%) had been exposed to lead at work. Out of these, 23 cases of ALS were linked to military service, which was believed to expose them to lead. Four instances featured high-intensity exposure, whereas 32 out of 36 cases had low-intensity exposure, with a median period of lead-exposed labor of 4 years (mean=11; range<1 to 45). Lead exposure at work did not affect the distribution of variables; however, ALS cases were more common in smokers and men, and C9orf72-positive cases were less common in lead-exposed people.
After 168 person-years of follow-up in the exposed group and 650 person-years in the non-exposed group, 11 (31%) of the 36 lead-exposed individuals achieved the survival outcome (PAV, tracheostomy, or death).
4. Assessing lead exposure in blood spots
4.1. Methodology
4.1.1. Preparation
To produce simulated blood spot standards, researchers added different amounts of lead into deionized water (DI) [6]. For the water standards, they used the same spotting procedure and supplies as for the blood. Typical blood spot cards showed that 150 microliters of ultrapure grade one deionized water contained 0, 5, 10, 50, and 100 micrograms of lead per deciliter (Fisher Scientific certified reference solution). The process was conducted in a sterile setting.
4.1.2. Statistic analysis
The researchers employed correlation and repeated measures analysis of variance to ascertain whether the spot sample size had an impact on the measurement outcomes. The consistency between the findings of the AAS and EDXRF measurements was investigated using linear regression and Pearson's correlation coefficient. At a 95% confidence level, statistical significance was established. The R 3.4.2 version was used for all analyses.
4.2. Results
Researchers spotted the identical samples on many cards with varying blood volumes to see how the spotting volume influenced the EDXRF test findings. We selected samples with lead concentrations of 7.1 and 8.8 micrograms per deciliter, respectively, using 75 (2 cards), 150 (7 cards), and 300 (5 cards) microliters of blood, as measured by ICP-MS. There was no discernible correlation between volume and the XRF detected signal when the EDXRF quantitative data was compared to the known ICP-MS concentrations and the known spotted sample quantities (p-value = 0.47, R = 0.21). Similarly, the repeated measures ANOVA revealed no change in volume (p-value = 0.40, F-value = 3.309), and neither sphericity nor normality were violated in these data.
5. The potential health risks of lead exposure in all periods
There is growing evidence from both preclinical and population-based studies linking developmental lead exposure to mental illnesses such as schizophrenia, major depressive disorder, mood disorders, and attention deficit hyperactivity disorder [7]. It is estimated that schizophrenia (SZ), a debilitating mental illness with an unknown cause that affects 1% of the world's population, costs society $155.7 billion annually. According to the agreement of scientists, SZ is a neurodevelopmental illness in which the phenotype occurs later in life due to a combination of genetic and environmental variables. Usually manifesting in late teens or early adulthood, SZ causes chronic impairment.
Affective flattening, alogia, avolition, and social withdrawal are examples of negative symptoms, whereas cognitive deficiencies are the hallmarks of SZ. Positive symptoms include delusions, hallucinations, and thought disorder. Cognitive impairments include working memory, attention, long-term memory, and cognitive flexibility deficiencies, and they frequently occur before favorable effects appear. These are factors that influence the intensity and course of the condition.
The first evidence of a link between chronic early-life lead exposure (CELLE) and SZ has come from population-based investigations, as reported by Opler and colleagues. Their study showed a strong association between prenatal lead exposure and a higher chance of developing SZ later, using two different large-scale population-based cohorts.
6. Early lead exposure is associated with molar hypomineralization
Asymmetrically distributed hypomineralized regions in the permanent first molars (PFM) are the hallmark of molar hypomineralization (MH), a common developmental enamel defect that may or may not affect the permanent incisors [8]. To better understand the impact of these developmental abnormalities and their underlying causes, oral health specialists have stepped up their research efforts in recent years. The prevalence of MH is thought to be between 13% and 14% worldwide, with South America having the highest rates at 18%. Yet, Mexican data show that 16% of fourth graders in public elementary schools suffer with MH, with the prevalence rising to 35% among children from low socioeconomic backgrounds—much higher than the global average.
6.1. Preparation
Each participant was given instructions to thoroughly brush their teeth with a toothbrush and regular toothpaste before the dental examination to get rid of dental plaque from the tooth surfaces. When there was substantial plaque or debris left on the tooth surfaces, the examiner used the participant's toothbrush to remove it. The study's end variable was the existence of Molar Hypomineralization (MH). The Molar Hypomineralization Assessment Criteria of the European Academy of Pediatric Dentistry (EAPD) were followed in the diagnosis of MH.
If at least one Porcelain Fused to Metal (PFM) surface had noticeable opacity or related clinical outcomes, like post-eruptive enamel degradation, atypical restorations, or tooth extraction because to MH, the participant was diagnosed with MH. When at least one PFM tooth surface satisfied the EAPD MH criterion, the person was deemed MH-positive.
6.2. Methodology
The presence of molar hypomineralization was assessed using the diagnostic standards provided by the European Academy of Pediatric Dentistry (EAPD) in a subset of children from the Early Life Exposure Study in Mexico, namely the environmental toxins (elements) cohort. Maternal blood lead levels and lead concentrations in the patella and tibia were measured during each trimester of pregnancy, and averages for each trimester were computed to determine prenatal lead exposure. The mother's blood lead levels at birth and the child's blood lead levels at 12 and 24 months of age were used to assess postnatal exposure.
6.3. Result
506 members of the ELEMENT cohort were recruited for the study, and 87 of them (17.2%) had a lead poisoning diagnosis. Although this link did not last into the first two years of postnatal life, the study found a strong correlation between maternal blood lead levels during pregnancy and the hypomineralization of deciduous teeth in children. Measurements of the lead levels in the mother's blood do not adequately account for the mobilization of lead stored in the mother's bone during pregnancy, which exposes the fetus to lead.
The discovery that leads produced from bone passes straight through the placental barrier without balancing with lead in red blood cells helps to explain this phenomenon. Blood led levels in the calcium-supplemented group and the placebo control group did not differ significantly in this study.
7. Discussion
According to the World Health Organization, lead and its compounds rank among the top ten most hazardous substances that have an impact on human health worldwide. Even though the causes of lead exposure have been determined, lead exposure from shooting ranges, for example, is gaining more attention in relation to the spread of lead in various places [9]. After the battery sector, this source is thought to be the second-largest source of lead pollution worldwide. Globally, shooting ranges emit between 10,000 and 60,000 tons of lead annually.
The problem of lead exposure at shooting ranges has been most well-documented in the United States; in Europe, it has received less attention, and Poland has not yet conducted any pertinent studies. For industrial studies, the research in South Korea has brought up several constraints [10]. First, monitoring of the work environment is done to gauge compliance. Instead of using random sampling, industrial hygienists choose the employees to be assessed using their own discretion, which is predicated on the highest possible exposure. The estimates obtained from these measurement values may be biased if workers are chosen using judgment sampling since it may be impossible to determine accuracy.
Although estimating based on the greatest possible exposure could result in overestimation, there is also a chance of underestimate because businesses bear the expense of workplace environment monitoring. Although they are unable to ascertain the combined impact of these factors, researchers believe that exposure levels are underestimated. Researchers think about examining only data over the detection limit to address the possible underestimating, but doing so carries the danger of overestimation and may only highlight high exposure levels.
8. Conclusion
To restate, the research on lead exposure is thoroughly summarized and synthesized in this article. Even while the existing approaches show a lot of improvement, they still need to be further improved. Future studies are expected to provide deeper understandings of the complex problems and fields of knowledge related to lead exposure as scientific and technology advancements progress.
References
[1]. Ji X, Wang B, Paudel YN, Li Z, Zhang S, Mou L, Liu K, Jin M. Protective Effect of Chlorogenic Acid and Its Analogues on Lead-Induced Developmental Neurotoxicity Through Modulating Oxidative Stress and Autophagy. Front Mol Biosci. 2021 Jun 11; 8: 655549. doi: 10.3389/fmolb.2021.655549. PMID: 34179077; PMCID: PMC8226318.
[2]. Zhang J, Su P, Xue C, Wang D, Zhao F, Shen X, Luo W. Lead Disrupts Mitochondrial Morphology and Function through Induction of ER Stress in Model of Neurotoxicity. Int J Mol Sci. 2022 Sep 28; 23(19): 11435. doi: 10.3390/ijms231911435. PMID: 36232745; PMCID: PMC9569474.
[3]. Virgolini MB, Aschner M. MOLECULAR MECHANISMS OF LEAD NEUROTOXICITY. Adv Neurotoxicol. 2021; 5: 159-213. doi: 10.1016/bs.ant.2020.11.002. Epub 2021 Feb 17. PMID: 34263090; PMCID: PMC8276936.
[4]. Choi J, Kim YS, Kim MH, Kim HJ, Yoon BE. Maternal lead exposure induces sex-dependent cerebellar glial alterations and repetitive behaviors. Front Cell Neurosci. 2022 Aug 22; 16: 954807. doi: 10.3389/fncel.2022.954807. PMID: 36072563; PMCID: PMC9442054.
[5]. Wang TW, Wuu J, Cooley A, Yeh TS, Benatar M, Weisskopf M. Occupational lead exposure and survival with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2023 Feb; 24(1-2): 100-107. doi: 10.1080/21678421.2022.2059379. Epub 2022 Apr 9. PMID: 35400246; PMCID: PMC9547984.
[6]. Specht AJ, Obrycki JF, Mazumdar M, Weisskopf MG. Feasibility of Lead Exposure Assessment in Blood Spots using Energy-Dispersive X-ray Fluorescence. Environ Sci Technol. 2021 Apr 20; 55(8): 5050-5055. doi: 10.1021/acs.est.0c06622. Epub 2021 Mar 24. PMID: 33759507; PMCID: PMC10615324.
[7]. Olufemi AC, Mji A, Mukhola MS. Potential Health Risks of Lead Exposure from Early Life through Later Life: Implications for Public Health Education. Int J Environ Res Public Health. 2022 Nov 30; 19(23): 16006. doi: 10.3390/ijerph192316006. PMID: 36498077; PMCID: PMC9741093.
[8]. Ahmed AT, Hector EC, Urena-Cirett JL, Mercado-Garcia A, Cantoral A, Hu H, Peterson KE, Tellez-Rojo MM, Martinez-Mier EA. Early Lead Exposure Associated with Molar Hypomineralization. Pediatr Dent. 2023 Sep 15; 45(5): 427-433. PMID: 37904269; PMCID: PMC10936227.
[9]. Darago A, Klimczak M, Jurewicz J, Kucharska M, Kilanowicz A. Assessment of lead exposure in indoor shooters in central Poland. Sci Rep. 2023 Aug 3; 13(1): 12605. doi: 10.1038/s41598-023-39847-3. PMID: 37537329; PMCID: PMC10400594.
[10]. Koh DH, Park JH, Lee SG, Kim HC, Jung H, Kim I, Choi S, Park D. Estimation of Lead Exposure Intensity by Industry Using Nationwide Exposure Databases in Korea. Saf Health Work. 2021 Dec; 12(4): 439-444. doi: 10.1016/j.shaw.2021.07.008. Epub 2021 Jul 17. PMID: 34900362; PMCID: PMC8640577.
Cite this article
Shuai,L. (2025). Lead Exposure under Varying Conditions and Environments. Theoretical and Natural Science,117,126-133.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ji X, Wang B, Paudel YN, Li Z, Zhang S, Mou L, Liu K, Jin M. Protective Effect of Chlorogenic Acid and Its Analogues on Lead-Induced Developmental Neurotoxicity Through Modulating Oxidative Stress and Autophagy. Front Mol Biosci. 2021 Jun 11; 8: 655549. doi: 10.3389/fmolb.2021.655549. PMID: 34179077; PMCID: PMC8226318.
[2]. Zhang J, Su P, Xue C, Wang D, Zhao F, Shen X, Luo W. Lead Disrupts Mitochondrial Morphology and Function through Induction of ER Stress in Model of Neurotoxicity. Int J Mol Sci. 2022 Sep 28; 23(19): 11435. doi: 10.3390/ijms231911435. PMID: 36232745; PMCID: PMC9569474.
[3]. Virgolini MB, Aschner M. MOLECULAR MECHANISMS OF LEAD NEUROTOXICITY. Adv Neurotoxicol. 2021; 5: 159-213. doi: 10.1016/bs.ant.2020.11.002. Epub 2021 Feb 17. PMID: 34263090; PMCID: PMC8276936.
[4]. Choi J, Kim YS, Kim MH, Kim HJ, Yoon BE. Maternal lead exposure induces sex-dependent cerebellar glial alterations and repetitive behaviors. Front Cell Neurosci. 2022 Aug 22; 16: 954807. doi: 10.3389/fncel.2022.954807. PMID: 36072563; PMCID: PMC9442054.
[5]. Wang TW, Wuu J, Cooley A, Yeh TS, Benatar M, Weisskopf M. Occupational lead exposure and survival with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2023 Feb; 24(1-2): 100-107. doi: 10.1080/21678421.2022.2059379. Epub 2022 Apr 9. PMID: 35400246; PMCID: PMC9547984.
[6]. Specht AJ, Obrycki JF, Mazumdar M, Weisskopf MG. Feasibility of Lead Exposure Assessment in Blood Spots using Energy-Dispersive X-ray Fluorescence. Environ Sci Technol. 2021 Apr 20; 55(8): 5050-5055. doi: 10.1021/acs.est.0c06622. Epub 2021 Mar 24. PMID: 33759507; PMCID: PMC10615324.
[7]. Olufemi AC, Mji A, Mukhola MS. Potential Health Risks of Lead Exposure from Early Life through Later Life: Implications for Public Health Education. Int J Environ Res Public Health. 2022 Nov 30; 19(23): 16006. doi: 10.3390/ijerph192316006. PMID: 36498077; PMCID: PMC9741093.
[8]. Ahmed AT, Hector EC, Urena-Cirett JL, Mercado-Garcia A, Cantoral A, Hu H, Peterson KE, Tellez-Rojo MM, Martinez-Mier EA. Early Lead Exposure Associated with Molar Hypomineralization. Pediatr Dent. 2023 Sep 15; 45(5): 427-433. PMID: 37904269; PMCID: PMC10936227.
[9]. Darago A, Klimczak M, Jurewicz J, Kucharska M, Kilanowicz A. Assessment of lead exposure in indoor shooters in central Poland. Sci Rep. 2023 Aug 3; 13(1): 12605. doi: 10.1038/s41598-023-39847-3. PMID: 37537329; PMCID: PMC10400594.
[10]. Koh DH, Park JH, Lee SG, Kim HC, Jung H, Kim I, Choi S, Park D. Estimation of Lead Exposure Intensity by Industry Using Nationwide Exposure Databases in Korea. Saf Health Work. 2021 Dec; 12(4): 439-444. doi: 10.1016/j.shaw.2021.07.008. Epub 2021 Jul 17. PMID: 34900362; PMCID: PMC8640577.









