1. Introduction
Drug delivery systems (DDS) are pivotal to effective therapeutic interventions in modern medicine. However, conventional delivery methods often face several challenges that limit their efficacy and compromise patient experience. For example, oral administration is hindered by low bioavailability due to degradation by gastric acid, digestive enzymes, and hepatic first-pass metabolism. The poor solubility and permeability of macromolecular drugs further impede intestinal absorption [1]. Additionally, traditional systems struggle to cross biological barriers, such as the blood-brain barrier and intestinal wall, limiting therapeutic options for neurological disorders and the oral delivery of biologics. Non-specific drug distribution remains a significant issue, contributing to off-target toxicity, particularly in chemotherapy, where healthy tissues often suffer alongside cancer cells. The need for frequent dosing to maintain therapeutic levels diminishes patient compliance, especially in chronic conditions, while individual variations in drug metabolism complicate the pursuit of personalized treatments [2].
Recently, microorganisms—including bacteria, viruses, and certain fungi—have emerged as promising natural drug carriers, offering solutions that overcome many of the limitations of traditional DDS. Their inherent biocompatibility reduces immune responses and tissue toxicity. In contrast to synthetic materials, microbial cell structures and metabolites are closely aligned with host biology, minimizing adverse reactions. Many microorganisms exhibit chemotaxis, enabling them to home in on diseased tissues such as tumors or inflamed areas, thereby enhancing local drug accumulation and reducing systemic exposure (Fig. 1). For instance, certain bacteria are capable of sensing hypoxia or acidity in tumor microenvironments and selectively colonizing these areas, improving targeting specificity. Additionally, their low immunogenicity and self-replicating properties enable sustained drug release, prolonging therapeutic effects and dynamically adjusting drug concentrations at lesion sites [2-4].
Advances in synthetic biology and genetic engineering have further expanded the applications of microorganisms in biomedicine. Tools such as CRISPR-Cas9 enable precise genome editing, enhancing the therapeutic potential of engineered microbes. These microbes can function as “micro-physicians,” autonomously diagnosing diseases, releasing drugs on demand, and self-destructing after treatment to minimize side effects. Synthetic biology also facilitates spatiotemporal control of drug delivery, enabling microbial chemotaxis pathways to direct microorganisms to tumor tissues and trigger site-specific drug release. Furthermore, microorganisms efficiently produce and deliver therapeutic agents, improving stability, bioavailability, and reducing production costs. However, concerns about biosafety and biocompatibility remain, and strategies such as gene-circuit-based kill switches and growth-restrictive mutations are being developed to address these challenges [5].
In summary, microbial carriers present a versatile platform for drug delivery, leveraging their biocompatibility, chemotaxis, low immunogenicity, and self-replication. When combined with synthetic biology and genetic engineering, these microorganisms offer a pathway to precise, sustained, and targeted therapies, advancing personalized medicine and enabling the treatment of complex diseases.
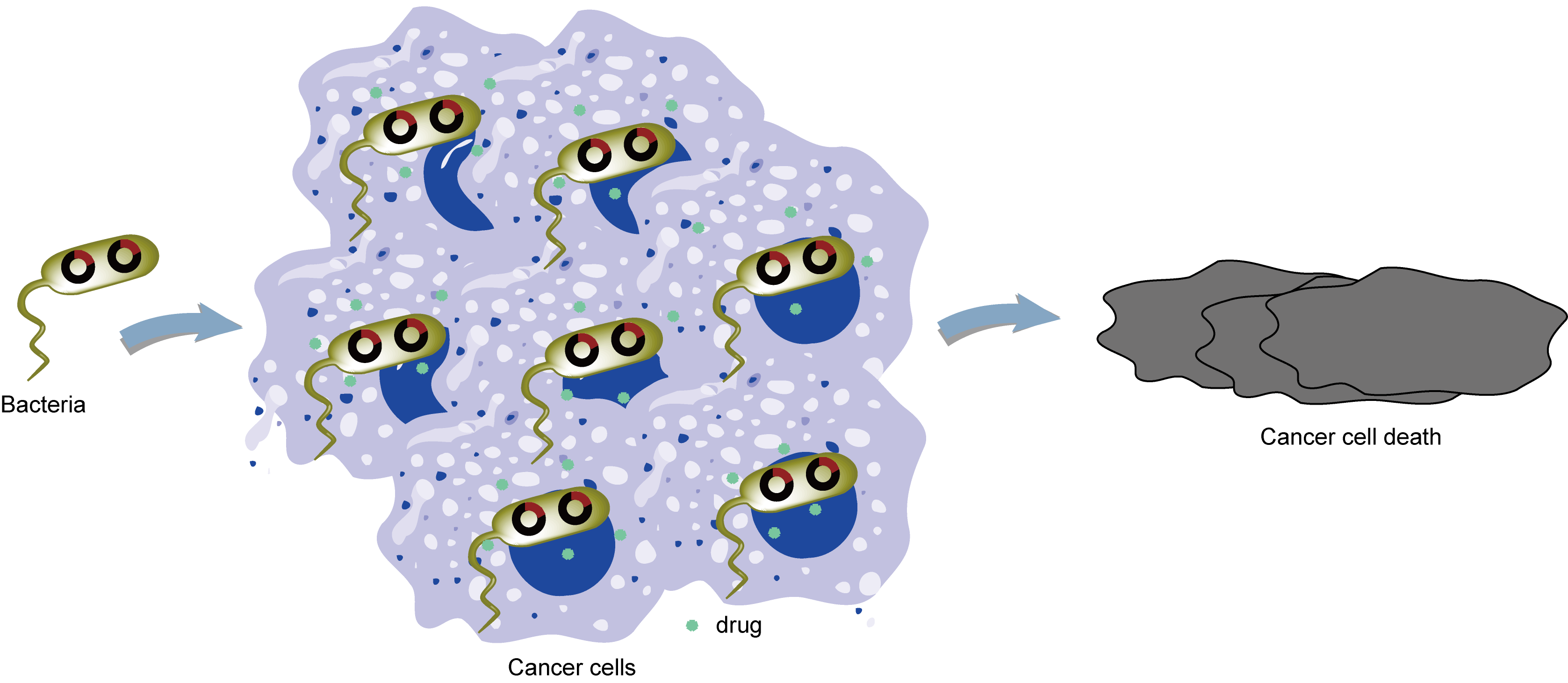
2. The basic principle of microorganisms as drug delivery carriers
2.1. Selection of microbial species
2.1.1. Probiotics
Probiotics, beneficial microorganisms found in the intestine, oral cavity, and skin, regulate microbiota, boost immune function, and inhibit pathogen colonization. Lactobacillus and Bifidobacterium, known for their acid tolerance and adhesion properties, facilitate long-term intestinal colonization and drug delivery. Their cell wall peptidoglycans and polysaccharides modulate immune responses. For instance, Lactobacillus acidophilus secretes IL-10 to mitigate inflammation, offering a potential therapy for inflammatory bowel disease (IBD). Genetically engineered probiotics, like Lactobacillus lactis, can sense intestinal signals and secrete IL-10, further enhancing IBD treatment [6,7].
2.1.2. Anaerobic bacteria
Anaerobic bacteria thrive in hypoxic environments and are commonly found in the intestines and oral cavity. These bacteria can adapt to low oxygen levels and specifically colonize hypoxic areas, such as tumors. For instance, Salmonella has been shown to colonize tumors and express anti-tumor drugs (e.g., TRAIL), effectively inhibiting tumor growth. Genetically engineered anaerobic bacteria serve as precise drug delivery systems, releasing therapeutic agents in tumor hypoxic regions. Modified Salmonella can be injected directly into host cells via the Type 3 secretion system (T3SS) to enhance delivery efficiency. Safety strategies, including self-destruct switches and restricted growth mutations, have been developed to mitigate risks [8,9].
2.1.3. Bacteriophage
Bacteriophages are viruses that specifically infect bacteria, offering high host specificity and the ability to accurately target and infect particular bacteria. Their outer shells can bind to bacterial cell walls, facilitating precise infection. Through phage surface display technology, bacteriophages can be engineered to carry therapeutic agents and deliver them to specific target cells or tissues, reducing distribution to non-target areas and minimizing side effects [10].
2.1.4. Synthetic microbes
Synthetic microbes are engineered with novel functions, such as targeted drug delivery and immune regulation, through tools like CRISPR-Cas9. These microbes can precisely target diseased tissues, releasing therapeutic agents at lesion sites, thus enhancing drug delivery and enabling gene therapy [11]. Microorganisms are increasingly used in the treatment of human diseases (Table 1).
|
Microbial species |
Application area |
Reference |
|
Engineered E. coli |
Using synthetic biology to make it secrete anti-inflammatory cytokines and other substances for the treatment of intestinal diseases |
[11] |
|
Lactobacillus |
Oral delivery carriers that tolerate stomach acid and release insulin or vaccine antigens in the gut. |
[8] |
|
Toxoid salmonella |
Genetically modified to reduce toxicity, it is used to deliver chemotherapy drugs or siRNA by taking advantage of its tendency to tumor hypoxic microenvironment. |
[9] |
|
Saccharomyces |
The surface displays the pathogen antigen and activates mucosal immunity and systemic immunity by oral or injection. |
[12] |
|
AAV |
Non-pathogenic viral vectors are widely used in genetic therapy of genetic diseases. |
[13] |
|
Bifidobacterium |
By expressing anticancer proteins or anti-inflammatory factors through genetic engineering, the natural intestinal colonization ability and low immunogenicity of these proteins can be used to deliver drugs to intestinal lesions . |
[8] |
|
Cyanobacteria |
Integrate photosensitive gene circuits to initiate drug synthesis under light to achieve precise time control of treatment. |
[14] |
2.2. Microbial targeting mechanism
2.2.1. Chemotaxis-oriented migration
Chemotaxis refers to the directed movement of microorganisms towards specific chemical signals. Many microorganisms exhibit chemotaxis, enabling them to migrate to lesion sites in response to chemical gradients in the host environment. For example, Salmonella can selectively migrate to tumor tissues by sensing low oxygen and acidic conditions within the tumor microenvironment [9,15].
2.2.2. Host tissue specific recognition
The interaction between microorganisms and host cells is crucial for targeted drug delivery. Adhesins and receptor-binding proteins on microbial surfaces interact with specific receptors or extracellular matrix components on host cells, ensuring precise localization to the lesion site. For instance, the capsule polysaccharide A (PSA) of Bacteroides fragilis can activate immune responses by binding to Toll-like receptors (TLR2/4) on host intestinal epithelial cells, exerting anti-inflammatory effects in models of ulcerative colitis [16].
2.2.3. Microenvironmental responsive drug release
Microbial drug delivery systems can release drugs in response to specific signals in the pathological microenvironment, such as pH changes, redox status, or enzyme activity. This mechanism enables precise drug release at lesion sites, increasing local drug concentration while minimizing side effects on healthy tissues. For instance, engineered Pseudomonas aeruginosa can sense quorum-sensing signals and release drugs automatically in the tumor microenvironment [10].
2.3. Drug loading strategy
2.3.1. Genetic engineering modification
Genetic engineering of microbial drug delivery enables microorganisms to synthesize, carry, transport, and target release drugs through gene editing and synthetic biology techniques. By utilizing technologies such as CRISPR-Cas9, microbial genetic engineering can ensure precise drug release in targeted areas and reduce side effects. For example, lambda bacteriophages deliver immune factors specifically to the tumor microenvironment by recombining and inserting the IL-12 gene, enhancing the killing effect of immune cells on tumors and significantly improving tumor immune responses, avoiding systemic side effects [17].
2.3.2. External encapsulation technology
External encapsulation technology involves the physical or chemical immobilization of therapeutic agents onto microorganisms or nanomaterials to enhance drug stability and delivery efficiency. Common methodologies include microencapsulation technology and nanocarrier-based drug encapsulation. Microencapsulation technology employs polymeric materials to encapsulate drugs into microparticulate systems, enhancing drug stability and prolonging release kinetics [18].Nanocarriers leverage the superior permeability of nanoparticles to encapsulate drugs either on the surface or within the internal matrix, enabling targeted delivery to specific sites. For instance, pH-sensitive mesoporous silica nanoparticles (MSNs) covalently conjugated to the surface of Lactococcus lactis have been demonstrated to achieve site-specific drug release in the gastrointestinal tract, significantly enhancing therapeutic efficacy [19].
2.3.3. Metabolic pathway engineering
Metabolic pathway design utilizes microbial metabolic processes to treat metabolites as therapeutic factors. Microorganisms produce various bioactive substances during their growth process, such as organic acids, enzymes, and vitamins, which have potential therapeutic effects. By finely regulating the metabolic pathways of microorganisms, therapeutic metabolites can be efficiently produced [20].For example, Lactobacillus reuteri upregulates the butyrate synthase gene through CRISPR activation technology, significantly increasing the synthesis and secretion of butyrate. Butyric acid, as a short chain fatty acid, can inhibit histone deacetylase (HDAC) activity and promote the expression of tumor suppressor gene p21 in the treatment of colorectal cancer, thereby inducing tumor cell differentiation and apoptosis [21].
3. Targeted drug delivery applications of microorganisms in different diseases
3.1. Tumor treatment
3.1.1. Application background
Microbial-targeted drug delivery systems are gaining increasing attention in tumor therapy. These systems exploit the natural tendencies of microorganisms to localize to the tumor microenvironment (TME) and deliver therapeutic molecules directly to the tumor site. Unlike passive targeting mechanisms of traditional nanocarriers, microorganisms actively recognize the TME through chemotaxis, metabolic adaptation, and gene regulation, thereby overcoming limitations such as the enhanced permeability and retention (EPR) effect. This active targeting significantly improves drug delivery accuracy and addresses challenges such as tumor immune escape and chemotherapy resistance [22].
3.1.2. Typical research cases
One notable advancement is the use of engineered Escherichia coli in the delivery of anti-tumor agents. Gene editing techniques allow E. coli to selectively colonize the TME and release therapeutic molecules. Escherichia coli Nissle 1917 (EcN), for example, can proliferate within the TME, secrete anti-tumor factors, and activate immune responses by blocking immune checkpoints (PD-L1 and CTLA-4) through nanobodies. This strategy reduces local tumor volume and inhibits metastasis. Additionally, EcN can regulate drug release through hypoxia-sensitive promoters or acidic pH-responsive elements, ensuring precise targeting while minimizing side effects on normal tissues . This immune synergistic mechanism enhances the killing effect on tumor cells and may form a lasting anti-tumor immune memory, providing new possibilities for long-term control and recurrence prevention of tumors [23,24].
3.2. Gene and immunotherapy
3.2.1. Background
The combination of gene editing and immunotherapy marks a new stage in precision medicine. Gene editing (such as CRISPR-Cas9) precisely modifies the genome, while immunotherapy activates the immune system's anti-tumor or anti infective abilities. Although individual applications have achieved results, tumor heterogeneity, immune escape, and off target toxicity remain challenges. In recent years, the use of engineered microorganisms for delivering therapeutic genes or antigens has become a new approach to overcome existing bottlenecks [11].
3.2.2. Engineering microbial delivery of CRISPR tools for gene repair
The engineered microbial delivery CRISPR system is an innovative gene editing technology that can accurately deliver gene editing tools such as CRISPR-Cas9 and single guide RNA (sgRNA) to the site of lesions [25].The natural chemotaxis and colonization ability of microorganisms enable them to release CRISPR components through cleavage programs under low pH, hypoxia, or specific metabolite stimulation, thereby mediating the knockout or repair of target genes. For example, in the treatment of ulcerative colitis (UC), researchers achieved targeted delivery at the site of colitis by introducing CRISPR-Cas9 and sgRNA plasmids into non pathogenic Escherichia coli. This' live biopharmaceutical 'can utilize the natural characteristics of microorganisms to achieve lesion targeting, avoiding the immunogenicity and off target effects caused by systemic administration, and providing long-lasting treatment [26].
4. Technical strategy and methods
4.1. Microbial engineering technology
4.1.1. Application of gene editing tools (CRISPR/Cas9)
Microbial engineering technology is a fundamental pillar of synthetic biology and metabolic engineering, which has made significant advancements in recent years, driven by the development of gene editing technologies. CRISPR/Cas9 has become a widely used genome editing tool due to its high efficiency, precision, and programmability. This system is derived from bacterial immune mechanisms, where it recognizes exogenous DNA and induces double-strand breaks, activating cell repair pathways to facilitate precise modification. Current research focuses on improving the accuracy and delivery efficiency of gene editing. To minimize off-target effects, high-fidelity Cas9 variants have been developed. In terms of delivery, the combination of bacteriophage particles and cationic liposomes has significantly enhanced transfection efficiency. Future advancements include the development of intelligent CRISPR systems that can respond to environmental signals and the integration of artificial intelligence to predict single-guide RNA (sgRNA) activity, enabling dynamic regulation of gene editing processes [25,26].
4.1.2. Metabolic pathway reconstruction and regulation
Metabolic pathway reconstruction and regulation are crucial strategies for optimizing microbial synthesis products, primarily involving the introduction, deletion, or replacement of gene modules, as well as dynamic regulation of gene expression and enzyme activity. Currently, whole-genome optimization utilizing CRISPR and other technologies allows for simultaneous editing of multiple metabolic nodes [26]. For example, engineered Escherichia coli Nissle 1917 was shown to increase drug production by 12.6 times by knocking out the pflB and ldhA genes, redirecting metabolic flow toward the butyric acid synthesis pathway. By combining promoter engineering, ribosome binding site (RBS) optimization, and quorum sensing systems (e.g., AHL feedback loops), the balance between product generation and cell growth can be improved. With the integration of genomics, metabolomics, and AI technology, metabolic engineering is progressing toward more intelligent systems [7].
4.1.3. Cell surface engineering
Cell surface engineering involves the precise modification of microbial membrane structures through gene editing or chemical modification to achieve targeted drug delivery, immune evasion, and environmental response functions. Core methods include: 1) utilizing self-transporters or S-layer proteins to display targeted ligands; 2) reshaping surface polysaccharide structures via glycosylation engineering to reduce immunogenicity; and 3) constructing environment-responsive membrane fusion proteins to enable controlled drug release [27]. For instance, the fusion of engineered E. coli EcN scFv with anti-EGFR single-chain antibodies and outer membrane protein OmpA enhanced tumor localization and facilitated synchronous chemotherapy drug release via pH-sensitive liposomes, resulting in an 82% reduction in tumor volume. By incorporating environmental response components, microorganisms can precisely release drugs in specific lesions, significantly improving treatment effectiveness [28].
4.2. Microbial targeting optimization strategy
4.2.1. Increased chemotaxis
Optimizing microbial chemotaxis genes (e.g., CheA, CheY) through gene editing can enhance microorganisms' response to specific chemical signals. For example, the chemotactic response of lactic acid bacteria to IL-8 was increased by 50%, improving their adaptability in complex environments. Furthermore, introducing stress-resistant genes can enhance the long-term survival of microorganisms in harsh environments such as the gastrointestinal tract [29].
4.2.2. Receptor specific modification
Genetic engineering can be used to modify microbial surfaces for specific recognition of target cells or tissues, improving targeting accuracy and reducing side effects. For example, fusing RGD peptides with bacterial outer membrane protein OmpA enables the recognition of integrins on the surface of tumor cells, allowing for localized therapeutic drug release. Additionally, enhancing the binding of lactic acid bacteria to immune cell receptors (such as TLR) can promote immune responses, playing a key role in combating infections and enhancing tumor immunotherapy [30].
4.3. Microbial monitoring and imaging technology
4.3.1. Fluorescence imaging technology
Fluorescence imaging technology is a critical tool for dynamic microbial monitoring and host-microbe interaction research. By employing gene editing techniques, fluorescent reporter genes (such as sfGFP, mCherry) can be integrated into microbial genomes, or exogenous fluorescent probes (e.g., Cy5, FITC) can be used to label microbial surfaces for real-time tracking of their physiological activities. This technology relies on the emission of fluorescence at specific wavelengths after excitation, with quantitative analysis conducted using optical systems. For instance, the sfGFP dual reporting system can monitor microbial behavior in the gut and its interaction with the host immune system in real-time. To overcome the issue of photobleaching, near-infrared fluorescent probes (e.g., Cy5.5) have been developed for real-time imaging of deep tissues [31,32].
4.3.2. Nanolabeling and tracking
Nanolabeling and tracking technologies offer higher sensitivity and specificity for microbial monitoring. Quantum dots, gold nanoparticles, and other nanomaterials can be combined with microorganisms for precise dynamic tracking. Quantum dots are particularly useful in microbial labeling and imaging due to their high fluorescence intensity, resistance to photobleaching, and multi-color emission characteristics. Gold nanoparticles enable highly sensitive optical detection via surface plasmon resonance (SPR). By combining quantum dots with labeled molecules, researchers have successfully achieved single-molecule-level monitoring of dormant proteins in bacteria, revealing the metabolic heterogeneity of antibiotic resistance. This technological breakthrough provides a new perspective on host-microbe interactions and highlights the potential of fluorescence imaging and nanolabeling technologies in disease treatment [33].
5. Challenges and future prospects
5.1. Current challenges
5.1.1. Biosafety
Microorganisms, such as engineered bacteria, bacteriophages, or probiotics, present unique advantages as drug delivery carriers, but their biosafety remains a critical challenge for clinical translation. Although most engineered microorganisms are attenuated or non-pathogenic strains, the exogenous elements they carry (such as plasmids or phage fragments) may trigger immune responses. Furthermore, genetically modified microorganisms may pose a risk of horizontal gene transfer, such as the spread of resistance genes. Additionally, microbial colonization could disrupt the host microbiota, potentially increasing the risk of cancer [34].
5.1.2. Drug delivery efficiency
The survival rate of microorganisms in complex physiological environments and the controllable spatiotemporal release of drugs remain major bottlenecks in their clinical applications. The physiological barriers and immune clearance mechanisms in the gastrointestinal tract pose significant challenges to microbial survival. For example, stomach acid and digestive enzymes can rapidly inactivate engineered bacteria, while the intestinal mucus layer and the host microbiota further limit microbial colonization. Existing environment-responsive gene circuits rely on a single biomarker, preventing highly specific drug release. Additionally, the host immune response can interfere with drug delivery efficiency [35].
5.1.3. Regulatory and ethical issues
Regulatory standards for microbial therapy have not yet been unified. Since microorganisms are considered "live drugs," the regulatory framework for traditional pharmaceuticals cannot be directly applied. Regulatory requirements for microbial therapies vary among countries, significantly increasing the cost of cross-border clinical trials. Additionally, deep modifications to microorganisms may spark ethical concerns related to "artificial life" and pose a risk of genetic contamination, particularly in tropical regions where ecological balance may be affected [36].
5.2. Future outlook
The integration of synthetic biology, nanotechnology, and artificial intelligence is driving the evolution of microbial-targeted drug delivery systems toward greater intelligence, precision, and programmability. Future research will focus on the dynamic regulation of intelligent microbial sensors, the development of personalized treatment systems, and the advanced application of synthetic biology tools.
Microbial sensors will overcome the limitations of traditional single-signal recognition, achieving precise drug release by integrating multimodal biomarkers such as metabolites and immune features. These sensor systems will enable real-time drug release based on changes in the tumor microenvironment, enhancing control over the timing and location of drug action.
Advances in synthetic biology have significantly improved the drug synthesis capabilities of microorganisms. By constructing orthogonal genetic code subsystems, microorganisms can co-produce multiple drugs without interfering with host cells. Biomimetic cell membrane technologies allow microorganisms to actively target and evade immune responses, expanding their potential applications in precision therapy.
Overall, microbial drug delivery systems are transitioning from the concept-validation phase to clinical applications. With continued technological innovation and interdisciplinary collaboration, microorganisms are expected to become "intelligent therapeutic agents" with autonomous decision-making capabilities, ushering in a new era of precision medicine.
The progress of synthetic biology has greatly enhanced the drug synthesis ability of microorganisms. By constructing orthogonal genetic code subsystems, microorganisms can achieve the co production of multiple drugs without interfering with host cells. Biomimetic cell membrane technology endows microorganisms with the ability to actively target and escape immunity, further expanding their potential applications in precision therapy.
Overall, microbial drug delivery systems are transitioning from the concept validation phase to clinical applications. In the future, with continuous technological innovation and interdisciplinary collaboration, microorganisms are expected to become "intelligent therapeutic agents" with autonomous decision-making capabilities, promoting precision medicine into a new era.
References
[1]. Tibbitt, M. W., Dahlman, J. E., & Langer, R. (2016). Emerging frontiers in drug delivery. Journal of the American Chemical Society, 138(3), 704-717.
[2]. Brayden, D.J., Mrsny, R.J., & Florence, A.T. (2018). Oral peptide delivery: Challenges and opportunities. Drug Delivery, 25(1), 23-45.
[3]. Hwang, I. Y., Lee, H. L., Huang, J. G., Lim, Y. Y., Yew, W. S., Lee, Y. S., & Chang, M. W. (2018). Engineering microbes for targeted strikes against human pathogens. Cellular and Molecular Life Sciences, 75, 2719-2733.
[4]. Riley, R. S., June, C. H., Langer, R., & Mitchell, M. J. (2019). Bacteria as potential vectors for gene therapy. Gene Therapy, 26(8), 273–281
[5]. Riglar, D. T., & Silver, P. A. (2018). Engineering bacteria for diagnostic and therapeutic applications. Nature Reviews Microbiology, 16(4), 214-225.
[6]. Suez, J., Zmora, N., Segal, E., & Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nature medicine, 25(5), 716-729.
[7]. Gurbatri, C. R., Lia, I., Vincent, R., Coker, C., Castro, S., Treuting, P. M., ... & Danino, T. (2020). Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Science translational medicine, 12(530), eaax0876.
[8]. Li, S. C., Hsu, W. F., Chang, J. S., & Shih, C. K. (2019). Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 cells. Nutrients, 11(5), 969.
[9]. Zheng, J. H., Nguyen, V. H., Jiang, S. N., Park, S. H., Tan, W., Hong, S. H., ... & Min, J. J. (2017). Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Science translational medicine, 9(376), eaak9537.
[10]. Pires, D. P., Cleto, S., Sillankorva, S., Azeredo, J., & Lu, T. K. (2016). Genetically engineered phages: a review of advances over the last decade. Microbiology and Molecular Biology Reviews, 80(3), 523-543.
[11]. Li, Y., Shen, X., Sun, X., et al. (2021). Research progress of CRISPR gene editing technology in microbial synthetic biology [J]. Synthetic Biology, 2(1), 106–120.
[12]. Kumar, R., & Kumar, P. (2019). Yeast-based vaccines: New perspective in vaccine development and application. FEMS yeast Research, 19(2), foz007.
[13]. Zhao, H., Li, Y., He, L., Pu, W., Yu, W., Li, Y., ... & Zhou, B. (2020). In vivo AAV-CRISPR/Cas9–mediated gene editing ameliorates atherosclerosis in familial hypercholesterolemia. Circulation, 141(1), 67-79.
[14]. Lee, M., & Woo, H. M. (2020). A logic NAND gate for controlling gene expression in a circadian rhythm in cyanobacteria. ACS Synthetic Biology, 9(12), 3210-3216.
[15]. SenGupta, S., Parent, C. A., & Bear, J. E. (2021). The principles of directed cell migration. Nature Reviews Molecular Cell Biology, 22(8), 529-547.
[16]. Round, J. L., Lee, S. M., Li, J., Tran, G., Jabri, B., Chatila, T. A., & Mazmanian, S. K. (2011). The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science, 332(6032), 974-977.
[17]. Alsaiari, S. K., Eshaghi, B., Du, B., Kanelli, M., Li, G., Wu, X., ... & Jaklenec, A. (2025). CRISPR–Cas9 delivery strategies for the modulation of immune and non-immune cells. Nature Reviews Materials, 10(1), 44-61.
[18]. Tomaro-Duchesneau, C., Saha, S., Malhotra, M., Kahouli, I., & Prakash, S. (2013). Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: current status and future directions. Journal of pharmaceutics, 2013(1), 103527.
[19]. Zhang, Y., Yu, G., He, W., Zhou, J., Ma, G., & Tang, R. (2018). Microencapsulation of bacteria with pH-sensitive hybrid hydrogel for enhanced viability and targeted delivery. Journal of Functional Biomaterials, 9(1), 11.
[20]. Olofsson, L. E., & Bäckhed, F. (2022). The metabolic role and therapeutic potential of the microbiome. Endocrine reviews, 43(5), 907-926.
[21]. Sun, X., & Zhu, M. J. (2018). Butyrate inhibits indices of colorectal carcinogenesis via enhancing α‐ketoglutarate‐dependent DNA demethylation of mismatch repair genes. Molecular nutrition & food research, 62(10), 1700932.
[22]. Huang, X., Guo, H., Wang, L., & Shao, Z. (2022). Engineered microorganism-based delivery systems for targeted cancer therapy: a narrative review. Biomaterials Translational, 3(3), 201.
[23]. Liu, W., Chen, W., Cai, Y., Tang, Y., & Chen, T. (2025). Advances in engineered bacteria vaccines for enhancing anti-cancer immunity. Microbiome Research Reports, 4(1), N-A.
[24]. Chen, H., Lei, P., Ji, H., Yang, Q., Peng, B., Ma, J., ... & Sun, D. (2023). Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies. Materials Today Bio, 18, 100543.
[25]. Feng, X., Li, Z., Liu, Y., Chen, D., & Zhou, Z. (2024). CRISPR/Cas9 technology for advancements in cancer immunotherapy: from uncovering regulatory mechanisms to therapeutic applications. Experimental Hematology & Oncology, 13(1), 102.
[26]. Anzalone, A. V., Koblan, L. W., & Liu, D. R. (2020). Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nature biotechnology, 38(7), 824-844.
[27]. Chen, Y., Liu, X., Zhang, Y., Wang, H., Li, Z., Mei, L., Zhao, J., & Chen, T. (2022). Engineered Escherichia coli Nissle 1917 with anti-EGFR scFv enhances tumor targeting and therapeutic efficacy. Science Advances, 8(15), eabm3682.
[28]. Rezaei Adriani, R., Mousavi Gargari, S. L., Bakherad, H., & Amani, J. (2023). Anti-EGFR bioengineered bacterial outer membrane vesicles as targeted immunotherapy candidate in triple-negative breast tumor murine model. Scientific Reports, 13(1), 16403.
[29]. Zhao, L., Yang, M., Zhang, M., et al. (2019). RGD peptide-conjugated Escherichia coli Nissle 1917 enhances tumor-targeted therapy through integrin αvβ3 recognition. ACS Synthetic Biology 8(6), 1215-1225.
[30]. Park, S. H., Zheng, J. H., Nguyen, V. H., Jiang, S. N., Kim, D. Y., Szardenings, M., ... & Min, J. J. (2016). RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics, 6(10), 1672.
[31]. Yoon, S. A., Park, S. Y., Cha, Y., Gopala, L., & Lee, M. H. (2021). Strategies of detecting bacteria using fluorescence-based dyes. Frontiers in chemistry, 9, 743923.
[32]. Li, S., Chen, L. X., Peng, X. H., Wang, C., Qin, B. Y., Tan, D., ... & Zhou, X. H. (2018). Overview of the reporter genes and reporter mouse models. Animal models and experimental medicine, 1(1), 29-35.
[33]. Abdel-Salam, M., Omran, B., Whitehead, K., & Baek, K. H. (2020). Superior properties and biomedical applications of microorganism-derived fluorescent quantum dots. Molecules, 25(19), 4486.
[34]. Shende, P., & Basarkar, V. (2019). Recent trends and advances in microbe-based drug delivery systems. DARU Journal of Pharmaceutical Sciences, 27, 799-809.
[35]. Yao, J., Zou, P., Cui, Y., Quan, L., Gao, C., Li, Z., ... & Yang, M. (2023). Recent advances in strategies to combat bacterial drug resistance: antimicrobial materials and drug delivery systems. Pharmaceutics, 15(4), 1188.
[36]. Rodriguez, J., Cordaillat-Simmons, M., Pot, B., & Druart, C. (2025). The regulatory framework for microbiome-based therapies: insights into European regulatory developments. npj Biofilms and Microbiomes, 11(1), 53..
Cite this article
Dong,X. (2025). Microorganisms as Vehicles for Targeted Drug Delivery: Applications and Prospects. Theoretical and Natural Science,114,72-82.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Extended Reality (XR) Applications in Medical Imaging
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Tibbitt, M. W., Dahlman, J. E., & Langer, R. (2016). Emerging frontiers in drug delivery. Journal of the American Chemical Society, 138(3), 704-717.
[2]. Brayden, D.J., Mrsny, R.J., & Florence, A.T. (2018). Oral peptide delivery: Challenges and opportunities. Drug Delivery, 25(1), 23-45.
[3]. Hwang, I. Y., Lee, H. L., Huang, J. G., Lim, Y. Y., Yew, W. S., Lee, Y. S., & Chang, M. W. (2018). Engineering microbes for targeted strikes against human pathogens. Cellular and Molecular Life Sciences, 75, 2719-2733.
[4]. Riley, R. S., June, C. H., Langer, R., & Mitchell, M. J. (2019). Bacteria as potential vectors for gene therapy. Gene Therapy, 26(8), 273–281
[5]. Riglar, D. T., & Silver, P. A. (2018). Engineering bacteria for diagnostic and therapeutic applications. Nature Reviews Microbiology, 16(4), 214-225.
[6]. Suez, J., Zmora, N., Segal, E., & Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nature medicine, 25(5), 716-729.
[7]. Gurbatri, C. R., Lia, I., Vincent, R., Coker, C., Castro, S., Treuting, P. M., ... & Danino, T. (2020). Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Science translational medicine, 12(530), eaax0876.
[8]. Li, S. C., Hsu, W. F., Chang, J. S., & Shih, C. K. (2019). Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 cells. Nutrients, 11(5), 969.
[9]. Zheng, J. H., Nguyen, V. H., Jiang, S. N., Park, S. H., Tan, W., Hong, S. H., ... & Min, J. J. (2017). Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Science translational medicine, 9(376), eaak9537.
[10]. Pires, D. P., Cleto, S., Sillankorva, S., Azeredo, J., & Lu, T. K. (2016). Genetically engineered phages: a review of advances over the last decade. Microbiology and Molecular Biology Reviews, 80(3), 523-543.
[11]. Li, Y., Shen, X., Sun, X., et al. (2021). Research progress of CRISPR gene editing technology in microbial synthetic biology [J]. Synthetic Biology, 2(1), 106–120.
[12]. Kumar, R., & Kumar, P. (2019). Yeast-based vaccines: New perspective in vaccine development and application. FEMS yeast Research, 19(2), foz007.
[13]. Zhao, H., Li, Y., He, L., Pu, W., Yu, W., Li, Y., ... & Zhou, B. (2020). In vivo AAV-CRISPR/Cas9–mediated gene editing ameliorates atherosclerosis in familial hypercholesterolemia. Circulation, 141(1), 67-79.
[14]. Lee, M., & Woo, H. M. (2020). A logic NAND gate for controlling gene expression in a circadian rhythm in cyanobacteria. ACS Synthetic Biology, 9(12), 3210-3216.
[15]. SenGupta, S., Parent, C. A., & Bear, J. E. (2021). The principles of directed cell migration. Nature Reviews Molecular Cell Biology, 22(8), 529-547.
[16]. Round, J. L., Lee, S. M., Li, J., Tran, G., Jabri, B., Chatila, T. A., & Mazmanian, S. K. (2011). The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science, 332(6032), 974-977.
[17]. Alsaiari, S. K., Eshaghi, B., Du, B., Kanelli, M., Li, G., Wu, X., ... & Jaklenec, A. (2025). CRISPR–Cas9 delivery strategies for the modulation of immune and non-immune cells. Nature Reviews Materials, 10(1), 44-61.
[18]. Tomaro-Duchesneau, C., Saha, S., Malhotra, M., Kahouli, I., & Prakash, S. (2013). Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: current status and future directions. Journal of pharmaceutics, 2013(1), 103527.
[19]. Zhang, Y., Yu, G., He, W., Zhou, J., Ma, G., & Tang, R. (2018). Microencapsulation of bacteria with pH-sensitive hybrid hydrogel for enhanced viability and targeted delivery. Journal of Functional Biomaterials, 9(1), 11.
[20]. Olofsson, L. E., & Bäckhed, F. (2022). The metabolic role and therapeutic potential of the microbiome. Endocrine reviews, 43(5), 907-926.
[21]. Sun, X., & Zhu, M. J. (2018). Butyrate inhibits indices of colorectal carcinogenesis via enhancing α‐ketoglutarate‐dependent DNA demethylation of mismatch repair genes. Molecular nutrition & food research, 62(10), 1700932.
[22]. Huang, X., Guo, H., Wang, L., & Shao, Z. (2022). Engineered microorganism-based delivery systems for targeted cancer therapy: a narrative review. Biomaterials Translational, 3(3), 201.
[23]. Liu, W., Chen, W., Cai, Y., Tang, Y., & Chen, T. (2025). Advances in engineered bacteria vaccines for enhancing anti-cancer immunity. Microbiome Research Reports, 4(1), N-A.
[24]. Chen, H., Lei, P., Ji, H., Yang, Q., Peng, B., Ma, J., ... & Sun, D. (2023). Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies. Materials Today Bio, 18, 100543.
[25]. Feng, X., Li, Z., Liu, Y., Chen, D., & Zhou, Z. (2024). CRISPR/Cas9 technology for advancements in cancer immunotherapy: from uncovering regulatory mechanisms to therapeutic applications. Experimental Hematology & Oncology, 13(1), 102.
[26]. Anzalone, A. V., Koblan, L. W., & Liu, D. R. (2020). Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nature biotechnology, 38(7), 824-844.
[27]. Chen, Y., Liu, X., Zhang, Y., Wang, H., Li, Z., Mei, L., Zhao, J., & Chen, T. (2022). Engineered Escherichia coli Nissle 1917 with anti-EGFR scFv enhances tumor targeting and therapeutic efficacy. Science Advances, 8(15), eabm3682.
[28]. Rezaei Adriani, R., Mousavi Gargari, S. L., Bakherad, H., & Amani, J. (2023). Anti-EGFR bioengineered bacterial outer membrane vesicles as targeted immunotherapy candidate in triple-negative breast tumor murine model. Scientific Reports, 13(1), 16403.
[29]. Zhao, L., Yang, M., Zhang, M., et al. (2019). RGD peptide-conjugated Escherichia coli Nissle 1917 enhances tumor-targeted therapy through integrin αvβ3 recognition. ACS Synthetic Biology 8(6), 1215-1225.
[30]. Park, S. H., Zheng, J. H., Nguyen, V. H., Jiang, S. N., Kim, D. Y., Szardenings, M., ... & Min, J. J. (2016). RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics, 6(10), 1672.
[31]. Yoon, S. A., Park, S. Y., Cha, Y., Gopala, L., & Lee, M. H. (2021). Strategies of detecting bacteria using fluorescence-based dyes. Frontiers in chemistry, 9, 743923.
[32]. Li, S., Chen, L. X., Peng, X. H., Wang, C., Qin, B. Y., Tan, D., ... & Zhou, X. H. (2018). Overview of the reporter genes and reporter mouse models. Animal models and experimental medicine, 1(1), 29-35.
[33]. Abdel-Salam, M., Omran, B., Whitehead, K., & Baek, K. H. (2020). Superior properties and biomedical applications of microorganism-derived fluorescent quantum dots. Molecules, 25(19), 4486.
[34]. Shende, P., & Basarkar, V. (2019). Recent trends and advances in microbe-based drug delivery systems. DARU Journal of Pharmaceutical Sciences, 27, 799-809.
[35]. Yao, J., Zou, P., Cui, Y., Quan, L., Gao, C., Li, Z., ... & Yang, M. (2023). Recent advances in strategies to combat bacterial drug resistance: antimicrobial materials and drug delivery systems. Pharmaceutics, 15(4), 1188.
[36]. Rodriguez, J., Cordaillat-Simmons, M., Pot, B., & Druart, C. (2025). The regulatory framework for microbiome-based therapies: insights into European regulatory developments. npj Biofilms and Microbiomes, 11(1), 53..









