1. Introduction
Exosomes are nanoscale extracellular vesicles characterized by a lipid bilayer membrane. They have diameters typically ranging from 40 to 160 nanometers and are originated from the endocytic pathway within cells [1]. Early endosomes undergo inward budding to form multi-vesicular bodies (MVBs), which subsequently fuse with the plasma membrane and release their intraluminal vesicles into the extracellular environment. Exosomes are widely distributed in various body fluids and primarily exhibit spherical morphology, such as flattened or cup-shaped forms. Their contents include cytoplasmic components, nuclear proteins or membrane proteins, mRNA, DNA and etc, making them essential intercellular communication mediators [2]. The application of exosomes in cancer therapy can be divided into two main research directions. First, studies have demonstrated that tumor cells are capable of releasing exosomes that carry tumor-specific antigens and other oncogenic factors. These exosomes play crucial roles in promoting immune tolerance, tumor progression, metastasis, and invasion. Therefore, inhibition of their secretion and distribution presents a novel strategy for anti-cancer therapy. Second, exosomes show many advantages such as drug delivery vehicles. Their phospholipid bilayer enables direct fusion with target cell membranes, thereby enhancing the efficiency of intracellular delivery. Additionally, due to their small size, exosomes can evade clearance by immune cells and promote deep penetration within tumor vasculature and the tumor microenvironment, which significantly improves drug bioavailability and therapeutic outcomes. This paper aims to comprehensively explore the current progress and technological developments in the use of engineered exosomes for cancer therapy. It will examine key challenges from exosome design and purification to functional application and assess their future clinical prospects in precision oncology.
2. Design and engineering of exosomes
In the development of exosomes as therapeutic delivery systems for cancer treatment, their design and engineering play a key role in ensuring clinical feasibility. Natural exosomes show many benefits, such as great biocompatibility, low immunogenicity, and the ability to penetrate cellular membranes. However, their intrinsic heterogeneity and limited targeting ability often fail to meet the precision medical requirements. Consequently, engineering strategies based on nanotechnology and synthetic biology have emerged as the mainstream approach for enhancing exosomal functionality and controllability.
2.1. Natural exosomes
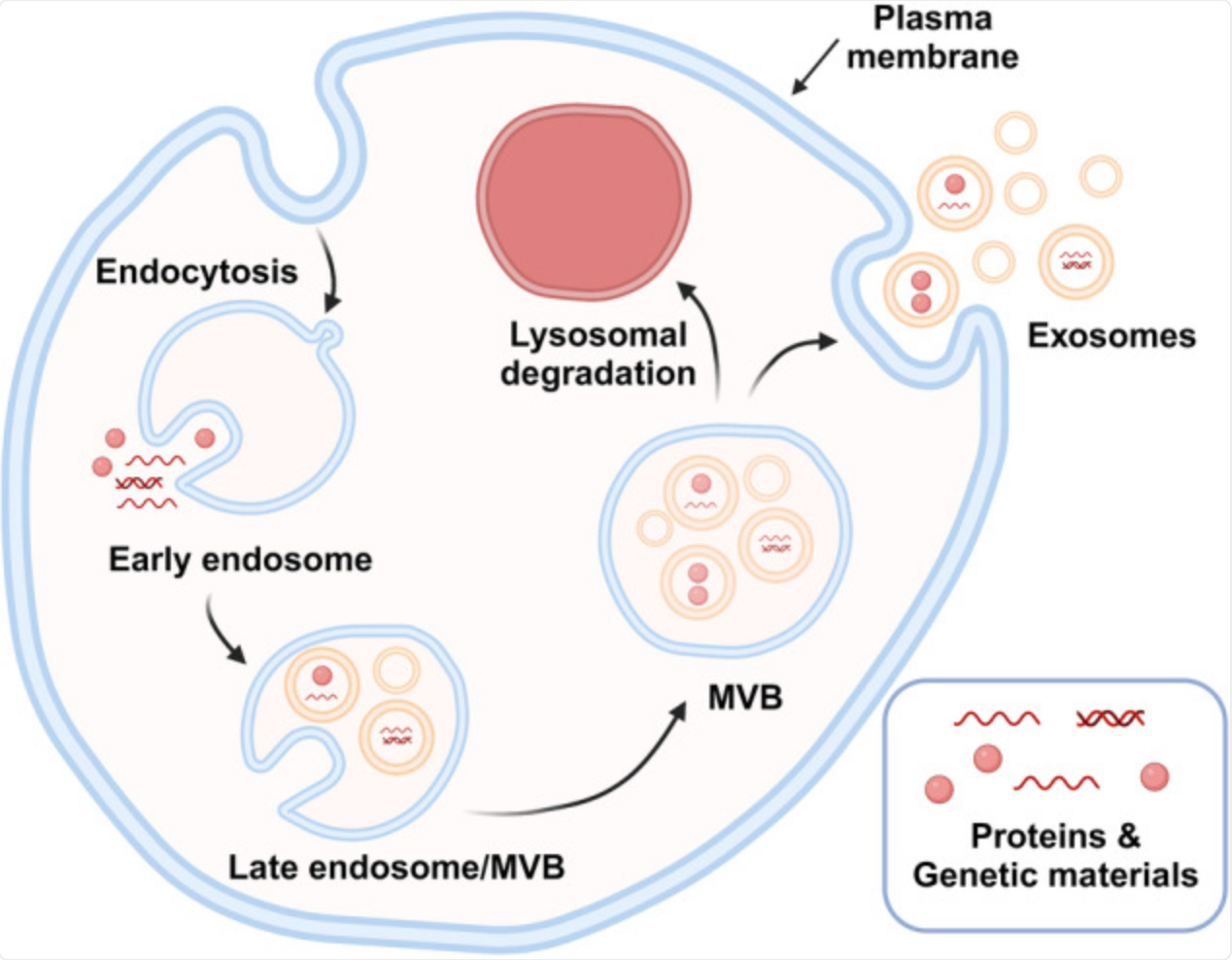
Exosome biogenesis is a complex, multistep process that occurs through the endosomal pathway, as illustrated in Fig. 1. It begins at the plasma membrane, where endocytosis forms early endosomes (EEs) through inward budding of the membrane [3]. Then, there are two possible pathways. EEs can either recycle back to the plasma membrane or continue to mature into MVBs, also called late endosomes. During this maturation, the limiting membrane of MVBs undergoes further inward budding, forming numerous intraluminal vesicles (ILVs) inside the MVBs, which encapsulate cytosolic components such as proteins and genetic materials [4].
MVBs will have 2 different pathways. First, they may fuse with lysosomes, leading to degradation of their cargo, or they may be directed toward the cell periphery for secretion [4]. In the secretory pathway, the small GTPases Rab27A and Rab27B (GTP-binding regulatory proteins, belonging to Rab family) mediate MVB trafficking to the plasma membrane. Finally, the soluble NSF attachment protein receptor (SNARE) complex facilitates membrane fusion, releasing ILVs into the extracellular space, where they are recognized as exosomes [3]. The composition and function of exosomes depend strongly on the cell of origin. For example, exosomes from dendritic cells can promote immune responses, while those from tumour cells often carry factors that ease metastasis. Therefore, selection of the donor cell type is critical when producing functionally tailored exosomes.
2.2. Engineering exosomes
To further confer therapeutic functions, engineering techniques are employed. It can be separated into 2 cargo loading methods: endogenous and exogenous. Each of them contains multiple different techniques, summarised [5].
Endogenous loading method focuses on parent cells. Usually, donor cells are transfected with plasmids or other constructs so that the resulting exosomes are able to carry drugs, including RNAs, miRNAs, proteins, small nucleic acids, and other chemotherapeutic drugs. The endogenous loading method includes two main techniques: cell- based co-incubation and transfection. In co-incubation, donor cells are cultured with drugs in the medium, allowing passive uptake and subsequent secretion of drug-loaded exosomes. This is simple but less controlled. In contrast, transfection delivers nucleic acids, proteins, or small molecules into donor cells via chemical reagents, electroporation, or viral vectors. This allows more precise and efficient cargo loading into exosomes [6]. Co-incubation is suited for small hydrophobic drugs like paclitaxel, while transfection is preferred for genetic material such as miRNAs or siRNAs [7].
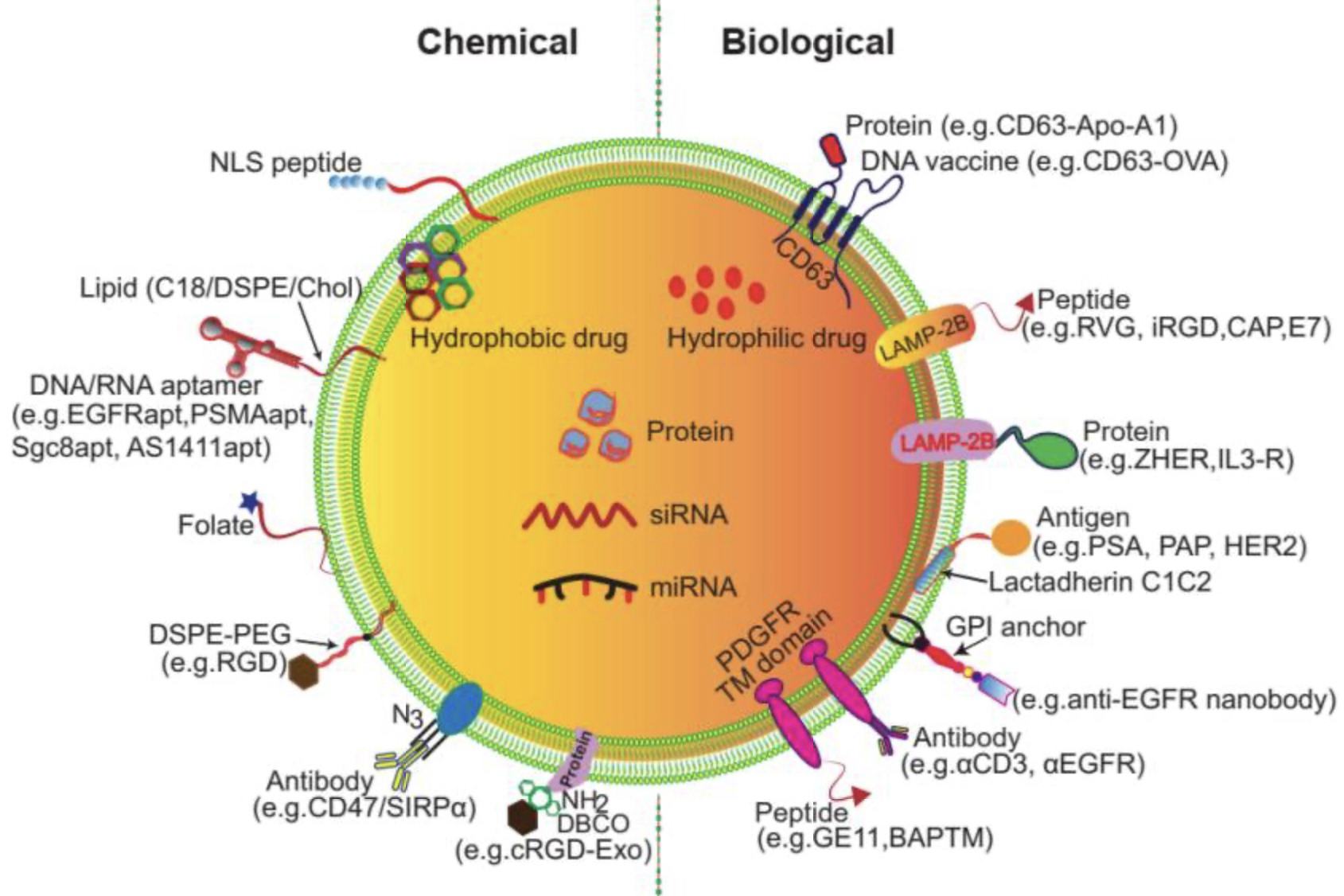
On the opposite side, exogenous loading methods tend to load drugs directly to the surface membrane of exosomes (Fig.2) [5]. It is functionalized through two main approaches: chemical and biological modification, with the key difference that chemical modification is applied to exosomes after secretion, while biological manipulation engineers the donor cells before secretion. Chemical modification attaches functional groups, such as peptides, proteins, or small molecules, to exosomal membranes through reactions with lipids or membrane proteins. For example, enabling binding to receptors like EGFR or HER2. Biological manipulation introduces targeting motifs or fusion proteins into donor cells through gene engineering. It results in exosomes that naturally express these proteins, such as iRGD, to improve tumour penetration [8]. Techniques used include cell-free co-incubation and transfection, sonication incubation, electroporation, freeze-thaw cycle, extrusion and saponin [5]. Different to endogenous, the co-incubation and transfection here involves purified exosomes, rely on direct interactions between drugs and exosomes, completely bypassing donor cells. Sonication applies ultrasonic waves to temporarily disrupt exosomal membranes, creating transient pores that facilitate drug entry while maintaining structural integrity after recovery. Electroporation uses short electrical pulses to induce membrane permeabilisation, enabling efficient encapsulation of charged molecules such as nucleic acids. The freeze–thaw method relies on repeated freezing and thawing to generate and reseal membrane fissures, allowing drugs to diffuse into the exosome lumen. Membrane extrusion forces exosomes and cargo through nano-porous membranes under pressure, producing uniformly sized, drug-loaded vesicles with improved stability [5,9]. Each of these techniques enables rapid, cell-independent loading with varying levels of efficiency and suitability for different cargo types.
3. Purification of exosomes
Following successful in vitro expression of engineered exosomes, the choice of purification method becomes a critical determinant of downstream applications. Given their small size and physical properties similar to other extracellular vesicles and protein aggregates, efficient and precise isolation remains a technical challenge.
Currently, ultracentrifugation (UC) is widely regarded as the standard procedure in most laboratories. But it is labor-intensive, time-consuming, and prone to sample loss. Therefore, the combination of UC and density gradient separation is often used to reduce large cellular debris and maximize exosome purification. However, the reproducibility of this method is often compromised due to variations in exosome density and morphology across different cell types and conditions. As an alternative, polymer-based precipitation methods offer ease of use, such as the polyethylene glycol (PEG)-induced sedimentation. However, they may typically yield lower purity due to co-isolated proteins and contaminants, making them less suitable for clinical or in vivo applications [10].
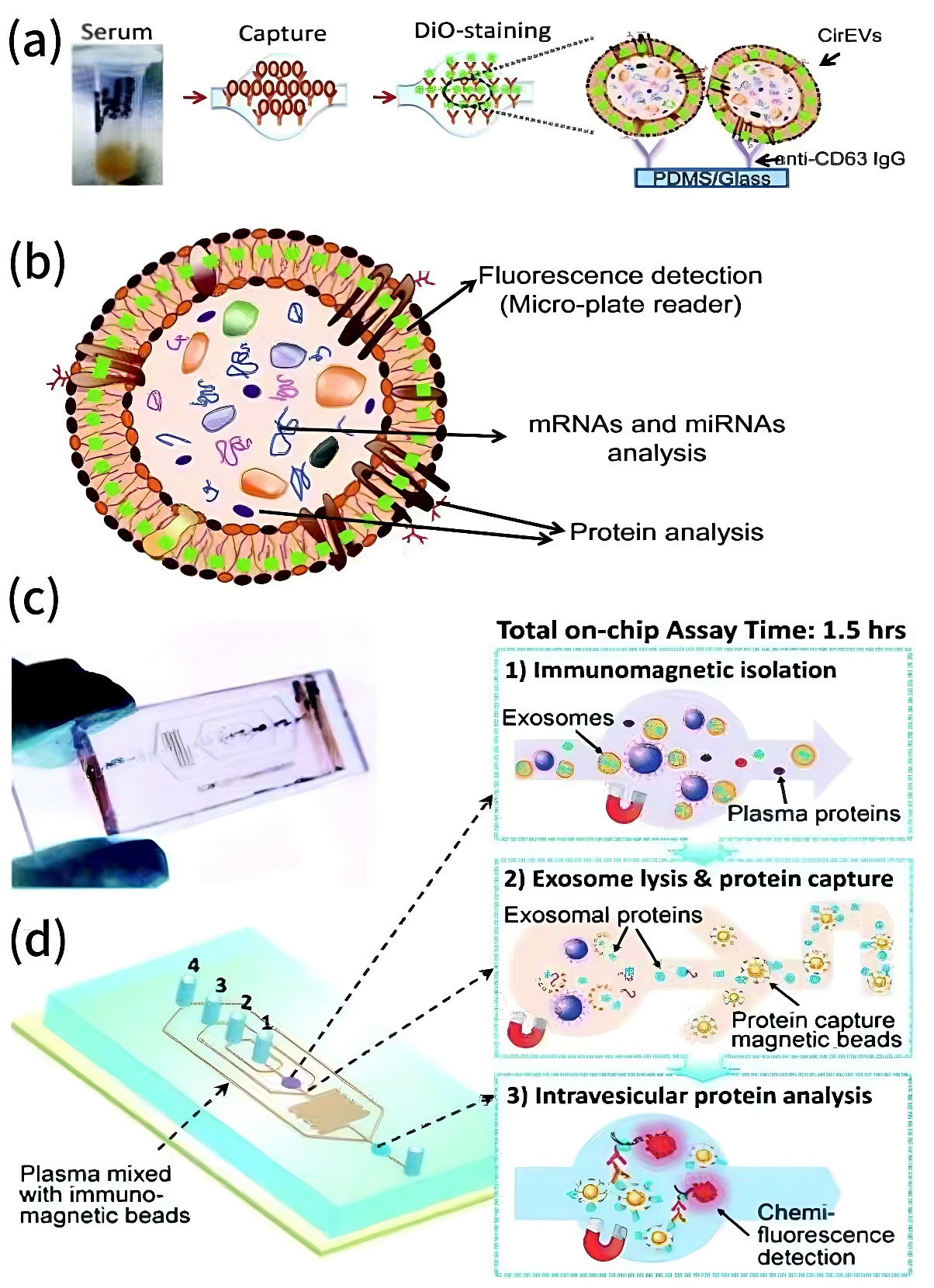
To overcome these limitations, innovative purification techniques have emerged. Nowadays, micro-fluidic platforms show outstanding advance. It can not only utilise nano-porous membranes, acoustic or electric field separation, but also enables high-throughput, automated exosome isolation with improved resolution. The top 2 examples are commercial immunochip (ExoChip) and integrate immunochip with detection, shown in Fig.3. ExoChip is coated with antibodies against CD63, a marker commonly found on exosome surfaces. When serum flows through the chip, exosomes bind to the immobilised antibodies and are trapped. Then, a fluorescent dye (DiO) is used to stain the captured exosomes, followed by plate reader to quantify them. ExoChip has many advantages, including commercial availability and standardisation, capable on small serum samples (~400 μL) and can captures intact exosomes with RNA for downstream analysis such as miRNA profiling. The integrated immunochip combines several steps together, including exosome capture with antibody-coated magnetic beads, enrichment of intra-vesicular proteins, and chemiluminescent detection. It requires only 30 μL of plasma, completes the process in under 100 minutes, and is particularly suited for protein biomarker analysis. These two platforms exemplify how microfluidic immunochips can integrate isolation, analysis, and diagnostic readout efficiently and with high clinical utility [11].
Once isolated, rigorous quality control is essential. Analytical tools such as dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) are commonly employed to determine particle size and concentration. Transmission electron microscopy (TEM) is used to confirm structural integrity, while Western blotting and ELISA assays are employed to the presence of characteristic surface proteins. To achieve inter-laboratory reproducibility and clinical-grade manufacturing, the establishment of standardised evaluation protocols and GMP-compliant production systems is imperative [10].
4. Applications of exosomes in oncology
Cancer is one of the most intensively studied areas for exosome-based research, encompassing both mechanistic insights and therapeutic innovation.
4.1. Molecular approaches
Increasing evidence shows that tumour cells actively release exosomes containing microRNAs (miRNAs), mRNAs, IncRNA, proteins, and other bioactive molecules that contribute to tumour growth, invasion, metastasis, and immune evasion [12]. Significant mechanism involves the exosome-mediated transfer of drug resistance traits. For example, efflux pump proteins like P-gp or inhibitory miRNAs are transmitted to adjacent tumour cells, inducing a phenomenon known as “bystander resistance,” which undermines chemotherapy efficacy. In response, various intervention strategies have been proposed to counteract exosome-mediated drug resistance. These include the use of neutral sphingomyelinase inhibitors (e.g., GW4869) to block exosome biogenesis or targeting exosome surface proteins with antibodies to prevent uptake by recipient cells [13]. More recently, engineered exosomes have been developed to act as “competitive inhibitors”. They deliver antagonistic miRNAs or siRNAs, such as miR-34a or members of the miR-200 family, to down regulate resistance-related genes and restore chemo-sensitivity in cancer cells [14].
4.2. Clinical approaches
The clinical translation of engineered exosomes is progressing rapidly, with multiple early-phase trials demonstrating their potential to enhance drug delivery and reduce systemic toxicity. For example, exosomes are modified to encapsulate small-molecule chemotherapeutics like paclitaxel (PTX). However, PTX suffers from poor solubility, systemic toxicity, allergic reactions, and lack of targeting, which limit its clinical use. Hybrid exosomes, created by fusing MSC-derived exosomes with folate-modified liposomes, were developed to overcome these drawbacks by combining the high biocompatibility and targeting ability of exosomes with the drug-loading efficiency and stability of liposomes. Exosomes have already shown promise in clinical trials for drug delivery due to their low immunogenicity, natural targeting, and ability to cross biological barriers, making them a safe and effective platform for next-generation drug delivery systems [7].
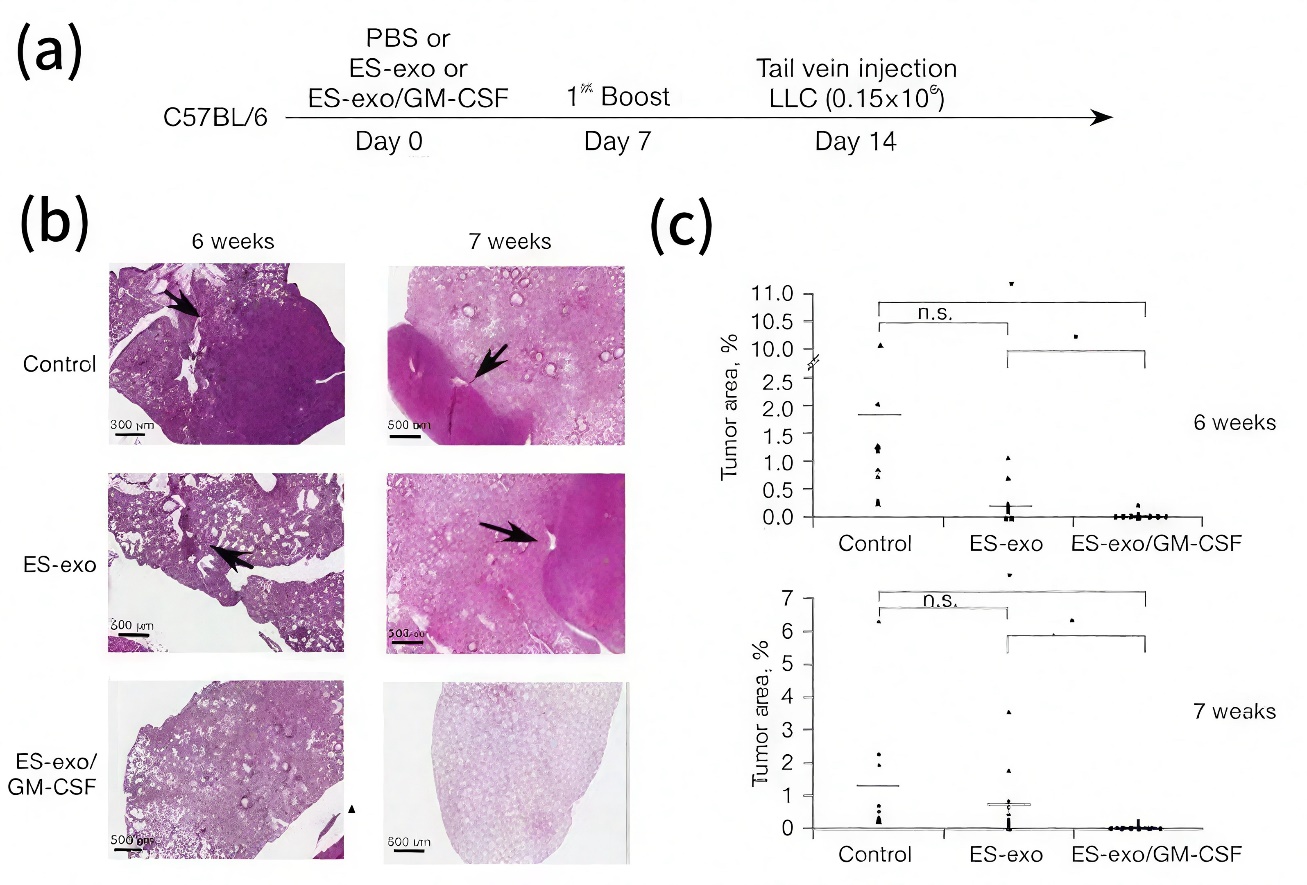
Moreover, exosome-based cancer vaccines carrying tumour-associated antigens are being developed to elicit anti-tumour immune responses. For example, ES-exo/GM-CSF vaccination was developed using exosomes derived from embryonic stem cells, engineered to express the immune-stimulatory cytokine GM-CSF. The vaccine was designed as a cell-free prophylactic strategy against metastatic lung cancer and was evaluated in a mouse model, as illustrated in Fig.4. Results demonstrated that vaccinated mice showed a significant reduction in lung tumour burden and metastatic lesions compared to controls. Mechanistically, the vaccine enhanced anti-tumour immunity by decreasing immunosuppressive cells, including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumour-associated macrophages, while increasing intramural CD8⁺ T cell cytokine production and B cell infiltration. This approach offers several advantages, including improved safety over live stem cell vaccines, potent immune activation, avoidance of teratoma formation, and the natural bioavailability and stability of exosome carriers, highlighting its potential as an effective and safe cancer immunotherapy platform [15].
These exploratory applications not only broaden the scope of exosome utility in oncology but also validate their role as a next-generation, multifunctional therapeutic platform.
5. Discussion
Despite exosomes have shown significant therapeutic potential, they still face a series of technical and ethical challenges. First, the heterogeneity and variability of exosome sources greatly influence their functional consistency, making it a central obstacle to large-scale production and clinical standardization. In particular, during industrial manufacturing, batch-to-batch variations in physical properties, loading capacity, and biological activity can be substantial. This increases the urgent need to establish standardized cell culture conditions, purification protocols and secretion induction strategies to ensure consistent product quality. At the same time, the pharmacokinetics of exosomes in vivo remain poorly understood, with most existing studies focusing on their enrichment in macrophage-rich organs such as the liver and spleen. Their distribution and clearance mechanisms in other tissues, particularly the central nervous system, remain unclear. In addition, while exosomes are natural products with inherently low immunogenicity, repeated administration or the loading of heterologous components may still elicit immune responses or adverse effects. This is particularly relevant in the context of long-term treatment or chronic disease management, where thorough safety assessments are imperative.
Future development efforts should focus on constructing intelligent and multifunctional exosome platforms. For example, combining nanomaterials, self-assembly techniques, and machine learning algorithms to develop “programmable exosomes” could enable precise recognition of specific tumor cells while simultaneously facilitating disease monitoring, diagnosis and therapy. Furthermore, the establishment of fully synthetic exosome-mimetic systems would provide novel solutions for scalable production and regulatory compliance, with the potential to significantly accelerate the transition of exosome-based therapies from laboratory research to clinical application.
6. Conclusion
This review comprehensively summarized recent advances in the design, purification, and application of engineered exosomes in tumor therapy, highlighting their potential as a next-generation therapeutic platform. We first discussed the biogenesis and natural characteristics of exosomes and outlined engineering strategies, including endogenous and exogenous cargo loading as well as surface functionalization, to improve their therapeutic performance and targeting specificity. Subsequently, we analyzed conventional and innovative purification technologies, emphasizing microfluidic immunochip platforms such as ExoChip and integrated immunochip. They have significantly enhanced exosome isolation efficiency and diagnostic capability. Finally, we reviewed the diverse applications of engineered exosomes in oncology. They can be used to overcome drug resistance, improve chemotherapeutic delivery, and serve as cancer vaccines. Notable examples include paclitaxel-loaded hybrid exosomes and ES-exo/GM-CSF vaccination. While engineered exosomes offer unique advantages such as biocompatibility, low immunogenicity, natural targeting, and multi-functionality, challenges such as production heterogeneity, standardization, and pharmacokinetic uncertainties remain to be addressed. Future work should focus on developing programmable, synthetic, and GMP-compliant exosome systems to ensure safety, scalability, and clinical translation. Overall, engineered exosomes represent a transformative innovation in precision oncology, enabling safer, more effective, and personalized tumor treatments. Their continued exploration and refinement are expected to accelerate their integration into clinical practice as a versatile and intelligent therapeutic platform.
References
[1]. Jiang, S., Liu, YH., Yu, Y., et al. Research progress on exosome drug delivery [J]. Chinese Medical Biotechnology, 2024, 19(2): 97-103. DOI: 10.3969/j.issn.1673-713X.2024.02.001.
[2]. Wei, YF., Fan, FY., Li, SL. & Zhang, KY. (2022). The Future of Exosomes and Mesenchymal Stem Cell-Derived Exosomes in the Diagnosis and Treatment of Parkinson’s Disease. Chinese Journal of Tissue Engineering Research, 26(25), 8.
[3]. Mashouri, L., Yousefi, H., Aref, A. R., Ahadi, A. M., Molaei, F., & Alahari, S. K. (2019). Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular cancer, 18(1), 75. https: //doi.org/10.1186/s12943-019-0991-5
[4]. Kim, H. I., Park, J., Zhu, Y., Wang, X., Han, Y., & Zhang, D. (2024). Recent advances in extracellular vesicles for therapeutic cargo delivery. Experimental & Molecular Medicine, 56(4), 836–849. https: //doi.org/10.1038/s12276-024-01201-6
[5]. Tian, J., Han, Z., Song, D., et al. (2023). Engineered exosome for drug delivery: Recent development and clinical applications. International Journal of Nanomedicine, 18, 7923–7940. https: //doi.org/10.2147/IJN.S444582
[6]. Bahadorani, M., Nasiri, M., Dellinger, K., Aravamudhan, S., & Zadegan, R. (2024). Engineering exosomes for therapeutic applications: Decoding biogenesis, content modification, and cargo loading strategies. International Journal of Nanomedicine, 19, 7137–7164. https: //doi.org/10.2147/IJN.S464249
[7]. Wang, X., Li, D., Li, G., et al. (2024). Enhanced therapeutic potential of hybrid exosomes loaded with paclitaxel for cancer therapy. International Journal of Molecular Sciences, 25(7), 3645. https: //doi.org/10.3390/ijms25073645
[8]. Liang, Y., Duan, L., Lu, J., & Xia, J. (2021). Engineering exosomes for targeted drug delivery. Theranostics, 11(7), 3183–3195. https: //doi.org/10.7150/thno.52570
[9]. Sadeghi, S., Tehrani, F. R., Tahmasebi, S., Shafiee, A., & Hashemi, S. M. (2023). Exosome engineering in cell therapy and drug delivery. Inflammopharmacology, 31(1), 145–169. https: //doi.org/10.1007/s10787-022-01115-7
[10]. Zhang, Y., Bi, J., Huang, J., Tang, Y., Du, S., & Li, P. (2020). Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. International Journal of Nanomedicine, 15, 6917–6934. https: //doi.org/10.2147/IJN.S264498
[11]. Li, P., Kaslan, M., Lee, S. H., Yao, J., & Gao, Z. (2017). Progress in exosome isolation techniques. Theranostics, 7(3), 789–804. https: //doi.org/10.7150/thno.18133
[12]. Zheng, Y., Jiang, W., Chen, D., Wang, L., Li, Y., Dai, L., Huang, L., & Wang, M. (2020). [Research progress on exosome in malignant tumors]. Zhongguo Fei Ai Za Zhi, 23(8), 689–694. https: //doi.org/10.3779/j.issn.1009-3419.2020.101.28
[13]. Barile, L., & Vassalli, G. (2017). Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & Therapeutics, 174, 63–78. https: //doi.org/10.1016/j.pharmthera.2017.02.020
[14]. Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.), 367(6478), eaau6977. https: //doi.org/10.1126/science.aau6977
[15]. Meng, S., Whitt, A. G., Stamp, B. F., Eaton, J. W., Li, C., & Yaddanapudi, K. (2023). Exosome-based cancer vaccine for prevention of lung cancer. Stem cell investigation, 10, 2. https: //doi.org/10.21037/sci-2022-030
Cite this article
Zhao,S. (2025). The Application of Engineered Exosomes in Tumour Therapy: Challenges and Prospects from Design to Clinic. Theoretical and Natural Science,124,48-56.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jiang, S., Liu, YH., Yu, Y., et al. Research progress on exosome drug delivery [J]. Chinese Medical Biotechnology, 2024, 19(2): 97-103. DOI: 10.3969/j.issn.1673-713X.2024.02.001.
[2]. Wei, YF., Fan, FY., Li, SL. & Zhang, KY. (2022). The Future of Exosomes and Mesenchymal Stem Cell-Derived Exosomes in the Diagnosis and Treatment of Parkinson’s Disease. Chinese Journal of Tissue Engineering Research, 26(25), 8.
[3]. Mashouri, L., Yousefi, H., Aref, A. R., Ahadi, A. M., Molaei, F., & Alahari, S. K. (2019). Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular cancer, 18(1), 75. https: //doi.org/10.1186/s12943-019-0991-5
[4]. Kim, H. I., Park, J., Zhu, Y., Wang, X., Han, Y., & Zhang, D. (2024). Recent advances in extracellular vesicles for therapeutic cargo delivery. Experimental & Molecular Medicine, 56(4), 836–849. https: //doi.org/10.1038/s12276-024-01201-6
[5]. Tian, J., Han, Z., Song, D., et al. (2023). Engineered exosome for drug delivery: Recent development and clinical applications. International Journal of Nanomedicine, 18, 7923–7940. https: //doi.org/10.2147/IJN.S444582
[6]. Bahadorani, M., Nasiri, M., Dellinger, K., Aravamudhan, S., & Zadegan, R. (2024). Engineering exosomes for therapeutic applications: Decoding biogenesis, content modification, and cargo loading strategies. International Journal of Nanomedicine, 19, 7137–7164. https: //doi.org/10.2147/IJN.S464249
[7]. Wang, X., Li, D., Li, G., et al. (2024). Enhanced therapeutic potential of hybrid exosomes loaded with paclitaxel for cancer therapy. International Journal of Molecular Sciences, 25(7), 3645. https: //doi.org/10.3390/ijms25073645
[8]. Liang, Y., Duan, L., Lu, J., & Xia, J. (2021). Engineering exosomes for targeted drug delivery. Theranostics, 11(7), 3183–3195. https: //doi.org/10.7150/thno.52570
[9]. Sadeghi, S., Tehrani, F. R., Tahmasebi, S., Shafiee, A., & Hashemi, S. M. (2023). Exosome engineering in cell therapy and drug delivery. Inflammopharmacology, 31(1), 145–169. https: //doi.org/10.1007/s10787-022-01115-7
[10]. Zhang, Y., Bi, J., Huang, J., Tang, Y., Du, S., & Li, P. (2020). Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. International Journal of Nanomedicine, 15, 6917–6934. https: //doi.org/10.2147/IJN.S264498
[11]. Li, P., Kaslan, M., Lee, S. H., Yao, J., & Gao, Z. (2017). Progress in exosome isolation techniques. Theranostics, 7(3), 789–804. https: //doi.org/10.7150/thno.18133
[12]. Zheng, Y., Jiang, W., Chen, D., Wang, L., Li, Y., Dai, L., Huang, L., & Wang, M. (2020). [Research progress on exosome in malignant tumors]. Zhongguo Fei Ai Za Zhi, 23(8), 689–694. https: //doi.org/10.3779/j.issn.1009-3419.2020.101.28
[13]. Barile, L., & Vassalli, G. (2017). Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & Therapeutics, 174, 63–78. https: //doi.org/10.1016/j.pharmthera.2017.02.020
[14]. Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.), 367(6478), eaau6977. https: //doi.org/10.1126/science.aau6977
[15]. Meng, S., Whitt, A. G., Stamp, B. F., Eaton, J. W., Li, C., & Yaddanapudi, K. (2023). Exosome-based cancer vaccine for prevention of lung cancer. Stem cell investigation, 10, 2. https: //doi.org/10.21037/sci-2022-030









