1. Introduction
O-GlcNAc glycosylation is a unique form of monosaccharide post-translational modification characterized by a reversible, rapid, and dynamic cycling process. This modification is uniquely mediated by a single pair of enzymes: O-GlcNAc transferase (OGT), which attaches N-acetylglucosamine to target proteins, and O-GlcNAcase (OGA), which removes it. Currently, research on OGT—particularly its substrate recognition mechanisms—has progressed further than research on OGA. Notably, studies have found that OGA levels are significantly lower in recurrent hepatocellular carcinoma (HCC) tissues than in non-recurrent HCC, whereas OGT levels show no significant correlation with tumor recurrence. O-GlcNAc glycosylation plays a key regulatory role in diverse cellular processes including gene expression, signal transduction, immunity, and metabolism, and aberrant O-GlcNAc levels are strongly associated with diseases such as diabetes, cancers, cardiovascular disease, and neurodegenerative disorders. Mapping the specific O-GlcNAc modification sites on proteins is important for understanding the mechanisms of disease. In many types of malignant tumors, abnormally elevated O-GlcNAc modifications have been observed in tumor cells, tissues, and organs, and this phenomenon is considered an important hallmark of cancer cells. Such aberrant O-GlcNAc modifications can drive malignant behaviors of tumor cells, including uncontrolled proliferation, invasion, and metastasis. Since OGT is the sole enzyme responsible for adding O-GlcNAc to proteins, abnormal OGT expression is closely linked to dysregulated O-GlcNAc levels in tumors. This review synthesizes recent advances in understanding the roles and mechanisms of OGT-mediated O-GlcNAc modification in malignant tumors.
2. Research progress on the role of OGT in malignant tumors
2.1. Biological functions of OGT
O-GlcNAc transferase (OGT) is a highly conserved glycosyltransferase encoded on the X chromosome. It contains an N-terminal tetratricopeptide repeat (TPR) domain and a C-terminal catalytic region composed of three subdomains: the N-terminal catalytic domain (N-Cat), intervening domain (Int-D), and C-terminal catalytic domain (C-Cat) [1]. In humans, OGT exists in three isoforms: ncOGT, mOGT, and sOGT. It catalyzes the transfer of O-linked β-N-acetylglucosamine (O-GlcNAc) to serine or threonine residues on nuclear, cytoplasmic, and mitochondrial proteins via a sequential bi–bi mechanism. Substrate recognition is primarily mediated by the TPR domain.
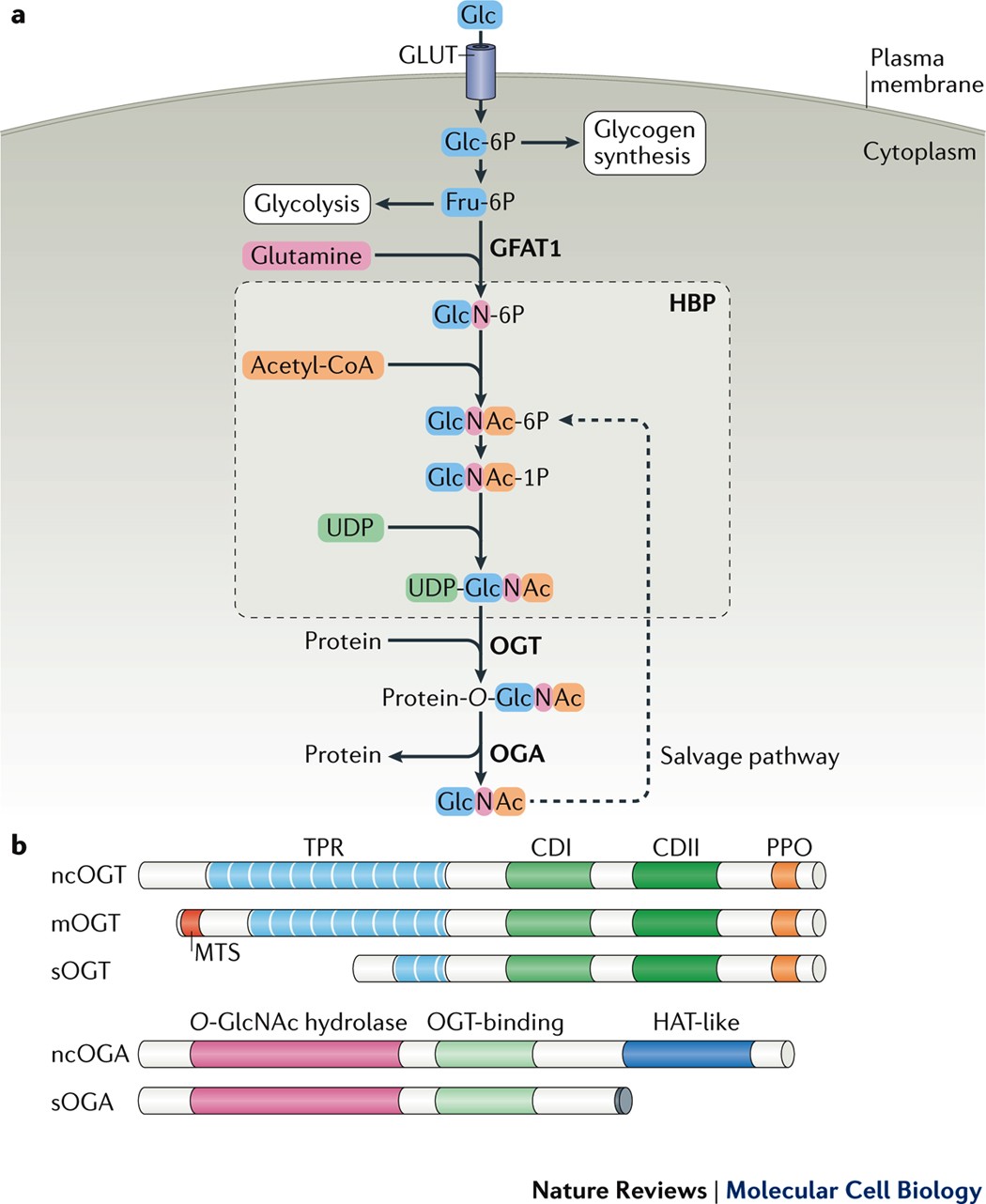
OGT is essential for mammalian development and cellular homeostasis. It is abundantly expressed in the brain and lymphoid tissues. Complete knockout of OGT results in embryonic lethality in mice. A well-known substrate, HCFC1, requires O-GlcNAcylation at Thr11 for proper proteolytic maturation and cell-cycle regulation. OGT also modulates cellular stress responses by translocating to the cytoplasm and mitochondria, where it modifies key signaling proteins. Notably, OGT affects mitotic progression by regulating CDK1 levels and activity [3-5]. In neurons, OGT is enriched at the postsynaptic density, and recent studies have linked OGT mutations to X-linked intellectual disability (XLID). These mutations impair glycosylation of HCFC1, compromise protein stability, and disrupt neurodevelopmental processes. OGT is frequently upregulated in diverse cancers, including breast, prostate, pancreatic, colorectal, and lung cancers. Elevated OGT levels are closely associated with aggressive tumor behavior. For instance, in breast cancer, transforming growth factor-β1 (TGF-β1) enhances O-GlcNAcylation of the chromatin remodeler MORC2, promoting migration and invasion. In prostate cancer, O-GlcNAcylation near Ser62 stabilizes MYC by inhibiting its phosphorylation-dependent degradation. In bladder cancer, urinary OGT levels are significantly increased in late-stage patients, indicating its potential as a diagnostic biomarker.
OGT also supports cancer stem-like properties. Its knockdown in breast cancer stem cells reduces sphere formation and decreases expression of stemness markers. In hepatocellular carcinoma, O-GlcNAcylation enhances the stability of transcription factors such as E2F, promoting invasiveness. In colorectal cancer, OGT expression is significantly elevated in tumor tissues compared to adjacent normal tissues, particularly under hypoxia or nutrient-deprived conditions. Collectively, these findings highlight OGT as a central regulator of tumorigenesis and a promising target for cancer diagnostics and therapeutics.
2.2. OGT and O-GlcNAc glycosylation
O-GlcNAc glycosylation is involved in many vital physiological processes, including cellular metabolism, other post-translational modifications, protein stability, and gene expression. As the sole enzyme that installs O-GlcNAc modifications on proteins, OGT is inherently tied to all biological processes regulated by O-GlcNAc. Studies on the pathogenesis of various diseases have demonstrated that severe or prolonged aberrant OGT expression disrupts O-GlcNAc homeostasis, which in turn contributes to the progression of numerous diseases.
Aberrant overexpression of OGT is often accompanied by elevated O-GlcNAc levels in disease states [6]. For instance, in diabetes, certain mitochondrial and insulin signaling proteins show abnormally high OGT expression along with increased O-GlcNAc modification. In the vast majority of solid tumors, OGT overexpression leads to higher global O-GlcNAc levels, resulting in a significant increase in tumor cell growth, proliferation, and metastatic potential. Immunohistochemical analyses have shown that breast tumor tissues have higher OGT and O-GlcNAc levels than adjacent normal tissues, and metastatic breast tumors exhibit even higher O-GlcNAc levels than primary tumors. In patients with liver cirrhosis and hepatocellular carcinoma, serum OGT levels are much higher than in healthy individuals, indicating that elevated OGT can signal underlying liver lesions. Notably, chemoresistant cancer cells also display significantly increased OGT expression and O-GlcNAc modification.
Importantly, increased OGT expression is not the only perturbation that can drive disease. Insufficient OGT expression is also implicated in certain chronic conditions, particularly neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), which are characterized by progressive neuronal degeneration and cell death. In affected neurons of AD and PD patients, key proteins show markedly lower O-GlcNAc modification levels compared to those in normal neurons, and this loss of O-GlcNAc is thought to contribute to the formation of neurofibrillary tangles and amyloid-β plaques—the signature pathologies of AD. Hyperphosphorylation of tau protein is known to drive neurofibrillary tangle formation in AD. Notably, tau can also be modified by O-GlcNAc, and an inverse relationship exists between tau O-GlcNAcylation and tau phosphorylation: the more hyperphosphorylated tau becomes, the lower its O-GlcNAc modification level.
Based on the above evidence, it is clear that dysregulation of O-GlcNAc homeostasis caused by aberrant OGT expression plays a significant role in many diseases. This insight provides important ideas and strategies for developing novel therapeutic approaches.
2.3. OGT in multiple cancers
In prostate cancer, OGT is frequently overexpressed and correlates with poor prognosis [7]. Immunohistochemistry reveals elevated OGT and O-GlcNAc levels in tumor cells compared to adjacent normal epithelium. Silencing OGT suppresses proliferation and invasiveness in prostate cancer cell lines, and in vivo, it reduces bone metastasis. Clinically, patients with low OGT expression show better five-year disease-free survival than those with high expression.
In breast cancer, elevated OGT and O-GlcNAc levels promote tumor aggressiveness. Matrix metalloproteinase 2 (MMP-2), a key enzyme in extracellular matrix (ECM) degradation, is downregulated upon OGT silencing, leading to reduced proliferation and invasion. In mouse models, OGT knockout significantly limits lung metastasis. Notably, OGA mRNA levels positively correlate with tumor grade and lymph node metastasis. Deletion of OGT reduces O-GlcNAc levels and inhibits anchorage-independent growth. Additionally, O-GlcNAcylation of cofilin at Ser108 facilitates metastasis. Progesterone receptor (PR)-positive tumors show higher OGT expression than normal tissue, supporting its potential as a therapeutic target.
In colorectal cancer, primary tumor tissues exhibit markedly higher O-GlcNAc and OGT expression than normal colon tissue. Increased O-GlcNAc levels enhance cell growth, invasion, and metastatic potential. Metastatic cell lines (e.g., SW620) display higher O-GlcNAc levels than primary tumor-derived cells (e.g., SW480) [9]. High OGT levels are associated with poor prognosis. Mechanistically, integrin α5 (ITGA5), which promotes epithelial–mesenchymal transition (EMT), is stabilized by O-GlcNAcylation, facilitating proliferation and inhibiting apoptosis. OGT thus promotes colorectal tumor progression by supporting cell survival.
In liver cancer, particularly hepatocellular carcinoma (HCC), O-GlcNAc levels are significantly elevated compared to normal liver tissue. In patients with HCC recurrence after liver transplantation, recurrent lesions exhibit markedly higher O-GlcNAcylation. In pancreatic ductal adenocarcinoma (PDAC), OGT is upregulated. O-GlcNAcylation of NF-κB p65 and its upstream kinases (IKKα/β) enhances NF-κB transcriptional activity, thereby promoting proliferation, reducing apoptosis, and increasing migration.
In bladder cancer, OGT mRNA is detectable in patients' urine but absent in healthy individuals. Higher urinary OGT levels correlate with advanced tumor grade and stage. Elevated OGT expression in muscle-invasive bladder cancer is associated with greater proliferation and chemoresistance. Knockout of OGT induces apoptosis, autophagy, and cell-cycle arrest, thereby sensitizing cancer cells to therapy.
In gastric cancer, OGT mRNA is upregulated in tumor tissues. Silencing OGT inhibits tumor growth in nude mice and induces apoptosis in vitro. Its expression correlates with malignancy grade, especially in cases with lymph node metastasis.
In summary, OGT overexpression is a common feature across multiple cancer types and contributes to malignant behaviors including proliferation, invasion, metastasis, and resistance to apoptosis. These findings support the therapeutic potential of targeting OGT. Several small-molecule OGT inhibitors have been developed. For instance, Ac-5SGlcNAc reduces O-GlcNAcylation of oncogenic proteins such as MYC, HIF-1α, and FOXM1, promoting tumor cell apoptosis. Moreover, combined treatment with an OGT inhibitor and the PI3K inhibitor GDC-0941 enhances apoptosis in ovarian cancer models.
3. Advances in the role of O-GlcNAc glycosylation in malignant tumors
3.1. Overview of HBP-Mediated O-GlcNAc modification
Protein glycosylation, a major post-translational modification, regulates diverse physiological and pathological processes, including protein folding, trafficking, degradation, and immune signaling. In humans, over half of all proteins are glycosylated, especially membrane-bound and secreted proteins. Based on linkage types, glycosylation is categorized into O-linked, N-linked, C-mannosylation, and glycosylphosphatidylinositol (GPI) anchoring. Among these, O-linked and N-linked glycosylation are most common.
Unlike N-glycosylation, which occurs on Asn-X-Ser/Thr motifs, O-glycosylation targets the hydroxyl groups of serine, threonine, or hydroxylysine without requiring a consensus sequence. This makes O-glycosylation sites more diverse. One specific subtype—O-linked N-acetylglucosamine (O-GlcNAc) modification—occurs predominantly in the nucleus, cytoplasm, and mitochondria, and is absent from membrane or secreted proteins. O-GlcNAc is added as a single sugar unit that modulates protein activity and intracellular signaling. This dynamic and reversible process is uniquely controlled by two enzymes: O-GlcNAc transferase (OGT), which adds GlcNAc, and O-GlcNAcase (OGA), which removes it.
Cancer cells are characterized by uncontrolled proliferation and high metabolic demands. To sustain growth, they reprogram metabolic pathways—including through O-GlcNAcylation—to meet energy and biosynthetic needs. One such adaptation is the Warburg effect, wherein cells preferentially use aerobic glycolysis over oxidative phosphorylation, even in the presence of oxygen [10]. The O-GlcNAc modification, enabled by a rapid and responsive two-enzyme cycle, supports this metabolic plasticity and facilitates malignant behaviors such as proliferation, invasion, metastasis, and immune evasion.
High O-GlcNAc levels are a hallmark of many cancers. For example, reducing O-GlcNAcylation of FOXM1 in prostate cancer cells downregulates VEGF and impairs angiogenesis [7]. Elevated global O-GlcNAc levels are associated with poorer prognosis in prostate cancer. In breast cancer, both clinical samples and cell lines show significantly higher O-GlcNAc levels than normal controls, particularly in triple-negative subtypes, where O-GlcNAc levels correlate positively with tumor grade and invasiveness. Similarly, metastatic lesions in colorectal cancer exhibit higher O-GlcNAc levels than primary tumors.
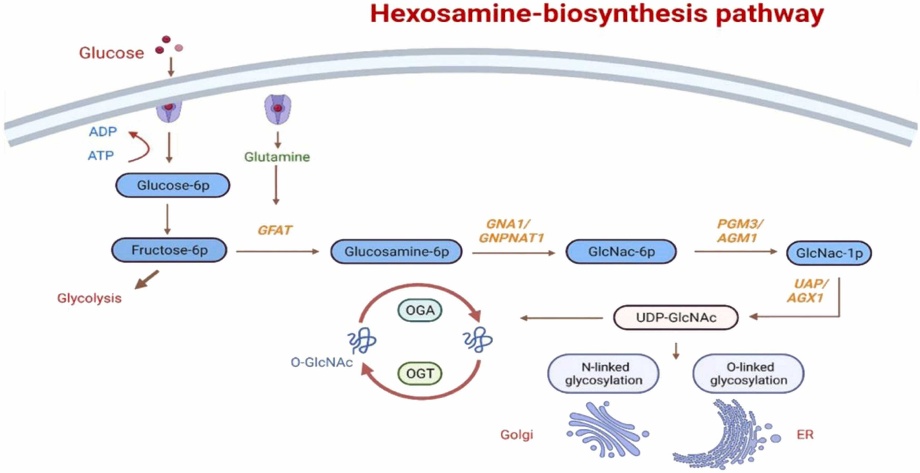
The hexosamine biosynthetic pathway (HBP) generates UDP-GlcNAc, the donor substrate for O-GlcNAcylation, and serves as a central metabolic integrator. HBP begins with glucose entering glycolysis: glucose is phosphorylated by hexokinase to glucose-6-phosphate (G6P), then converted to fructose-6-phosphate (F6P). The rate-limiting enzyme glutamine–fructose-6-phosphate amidotransferase (GFAT) transforms F6P into glucosamine-6-phosphate using glutamine. Subsequent steps involve GNA1 (acetylating to GlcNAc-6-P), AGM1 (isomerizing to GlcNAc-1-P), and UAP1 (producing UDP-GlcNAc).
UDP-GlcNAc levels dictate global O-GlcNAcylation status. HBP is highly sensitive to glucose availability: under low glucose, compensatory mechanisms maintain UDP-GlcNAc synthesis; under high glucose, excessive flux through HBP elevates O-GlcNAc levels. Besides nutrient sensing, HBP is responsive to stress and modulates endoplasmic reticulum and mitochondrial stress responses. Thus, O-GlcNAc modification functions as a key intracellular sensor of metabolic and environmental cues.
3.2. Role of O-GlcNAc modification in malignant tumors
3.2.1. O-GlcNAc modification promotes malignant biological behavior
O-GlcNAc modification is closely involved in essential cellular functions such as proliferation, signaling, gene regulation, and mitochondrial activity. Its dysregulation contributes to various pathological states. In cancer, elevated O-GlcNAc levels promote tumor cell survival and aggressiveness by altering proteins that drive hallmark traits, including angiogenesis, immune evasion, and resistance to cell death. Consistently, O-GlcNAcylation is significantly upregulated in tumor tissues—including liver, lung, colorectal, and pancreatic cancers—compared to normal counterparts [12,13].
(1) O-GlcNAc modification regulates gene expression.
Tumor cells adjust gene expression via O-GlcNAcylation in response to metabolic and environmental cues. This modification occurs at nearly all stages of transcription and affects multiple transcription factors regulated by RNA polymerase II. In HeLa cells, O-GlcNAc sites have been identified on histones H2A (Thr101), H2B (Ser36), and H4 (Ser47), predominantly within tail regions. It also modifies the C-terminal domain of RNA polymerase II, facilitating pre-initiation complex formation.
O-GlcNAcylation influences chromatin structure and histone crosstalk, interacting with acetylation and phosphorylation marks. These changes alter gene expression programs associated with malignancy.
(2) O-GlcNAc modifications regulate signaling pathways in tumor cells.
As a nutrient and stress sensor, O-GlcNAc modification fine-tunes signaling cascades that promote tumor progression. Complete OGT deletion is embryonically lethal in mice and Drosophila, underscoring its essential role in development.
O-GlcNAcylation modifies various kinases and phosphatases, altering key oncogenic pathways. In the Hippo pathway, phosphorylation of YAP at Ser127 typically inhibits its nuclear translocation. However, O-GlcNAcylation at Ser109 impairs this inhibitory phosphorylation, enabling YAP to activate pro-proliferative genes.
In pancreatic cancer, O-GlcNAcylation enhances NF-κB signaling. The p65 subunit and upstream kinases IKKα/β are modified, increasing NF-κB activity, suppressing apoptosis, and promoting metastasis. Additionally, O-GlcNAcylation of NF-κB enhances its acetylation, further amplifying transcriptional output of survival genes [14].
(3) O-GlcNAc modification of FOXA1 promotes tumor invasion and metastasis.
The pioneer transcription factor FOXA1 (also known as hepatocyte nuclear factor 3α) regulates chromatin accessibility and is implicated in hormone-responsive tumors. It cooperates with the androgen receptor in prostate cancer, enhances NKX2-1–dependent oncogene expression in lung cancer, and drives enhancer remodeling in pancreatic ductal adenocarcinoma.
In HER2-positive breast cancer, FOXA1 overexpression contributes to HER2 signaling, potentially via the ppGalNAc-T4–FOXA1–ERα axis. Multiple studies confirm that FOXA1 undergoes O-GlcNAcylation, which stabilizes the protein and enhances its transcriptional activity.
Importantly, O-GlcNAcylated FOXA1 suppresses E-cadherin transcription—a key epithelial marker and tumor suppressor. Loss of E-cadherin promotes invasion and metastasis. Thus, by repressing E-cadherin, O-GlcNAc-modified FOXA1 facilitates metastatic dissemination and tumor progression.
3.2.2. O-GlcNAc glycosylation mediates cellular metabolic dysregulation
Glucose metabolism is central to cellular energy production. After entering cells via glucose transporters (GLUTs), glucose is processed through glycolysis, the pentose phosphate pathway (PPP), and the tricarboxylic acid (TCA) cycle to generate ATP [15]. Tumor cells exhibit a markedly elevated glycolytic rate—approximately 20–30 times that of normal cells—and rely heavily on this pathway to support their rapid growth and invasion.
Under normoxic conditions, differentiated cells oxidize glucose via mitochondrial respiration, yielding ~36 ATP per glucose. In contrast, cancer cells preferentially convert glucose to lactate even in the presence of oxygen, a phenomenon known as the Warburg effect. Although glycolysis is less efficient in ATP yield, it generates energy and biosynthetic precursors more rapidly, helping tumor cells maintain high ATP/ADP and NADPH/NAD⁺ ratios, tolerate hypoxic microenvironments, and sustain anabolic demands for proteins, nucleic acids, and lipids. Moreover, lactate accumulation promotes angiogenesis, immune evasion, and metastasis, thereby conferring a survival advantage.
Emerging evidence supports that cancer progression is tightly coupled to dysregulated metabolism, in which protein glycosylation, particularly O-GlcNAcylation, serves as a critical mediator linking metabolic signals and oncogenic pathways.
For example, hypoxia-inducible factor 1α (HIF-1α) enhances glycolysis and angiogenesis under hypoxia by upregulating target genes such as GLUT1. O-GlcNAcylation stabilizes HIF-1α, promoting its transcriptional activity, while reducing O-GlcNAc levels accelerates its degradation and suppresses glycolysis. Similarly, the key glycolytic enzyme phosphofructokinase-1 (PFK-1) is activated via O-GlcNAcylation in lung cancer cells, increasing glycolytic flux and redirecting glucose toward the PPP, thereby supporting redox balance and proliferation.
O-GlcNAcylation also affects glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the PPP. Under hypoxic stress, G6PD activation by O-GlcNAcylation enhances NADPH production and biosynthesis, sustaining tumor growth. In addition, hypersialylated glycans on the tumor cell surface—often coordinated with altered glycosylation machinery—facilitate immune escape by triggering immunosuppressive signaling.
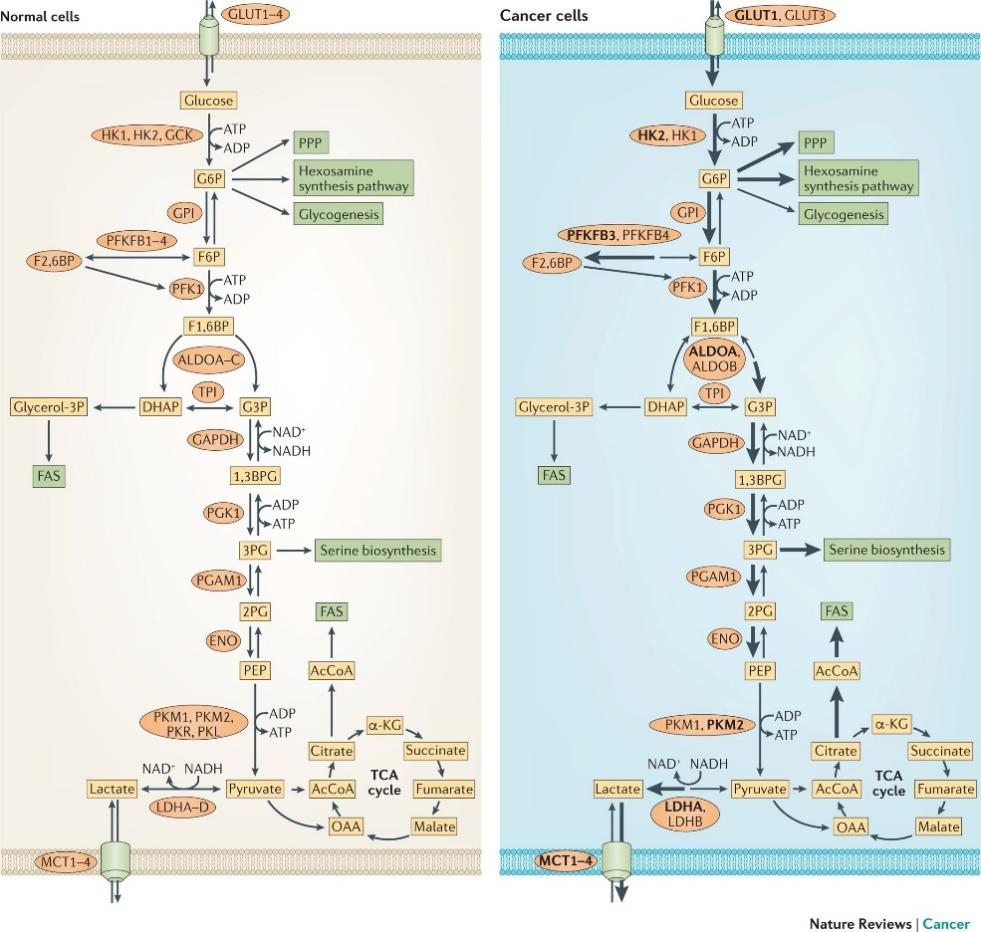
Together, these findings indicate that O-GlcNAcylation orchestrates key metabolic and signaling pathways to reprogram glucose utilization and promote tumorigenic adaptation.
4. Conclusion and outlook
In summary, O-GlcNAc glycosylation—catalyzed by O-GlcNAc transferase (OGT)—emerges as a crucial post-translational modification involved in regulating cancer-related processes such as aberrant proliferation, angiogenesis, epithelial–mesenchymal transition (EMT), immune evasion, metabolic reprogramming, and therapeutic resistance. Aberrant OGT expression and dysregulated O-GlcNAc homeostasis have been consistently observed in various malignancies, highlighting their diagnostic, prognostic, and therapeutic potential.
Despite significant advances, several critical questions remain unresolved. First, the precise regulatory network linking O-GlcNAcylation to specific oncogenic pathways remains incompletely mapped, especially in the context of tissue specificity and tumor heterogeneity. Second, although several small-molecule OGT inhibitors have shown promise in preclinical models, their specificity, bioavailability, and off-target effects require further optimization. Additionally, the dual role of O-GlcNAcylation in both tumor progression and neuroprotection underscores the need for targeted strategies that minimize collateral toxicity in normal tissues.
Future research should focus on integrating multi-omics approaches—such as glycoproteomics, single-cell transcriptomics, and spatial metabolomics—to uncover novel O-GlcNAc-modified targets and context-dependent regulatory mechanisms. The development of selective and reversible OGT modulators, in combination with conventional chemotherapies or immune checkpoint inhibitors, also holds great promise. Ultimately, a deeper mechanistic understanding of O-GlcNAc signaling in cancer may pave the way for more precise and effective therapeutic interventions.
References
[1]. Zhang N, Jiang H, Zhang K, et al. OGT as potential novel target: Structure, function and inhibitors. Chemico-Biological Interactions 2022; 357: 109886.
[2]. Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nature reviews Molecular cell biology 2017; 18(7): 452-65.
[3]. Itkonen HM, Gorad SS, Duveau DY, et al. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget 2016; 7(11): 12464.
[4]. Itkonen HM, Urbanucci A, Martin SE, et al. High OGT activity is essential for MYC-driven proliferation of prostate cancer cells. Theranostics 2019; 9(8): 2183.
[5]. Levine ZG, Potter SC, Joiner CM, et al. Mammalian cell proliferation requires noncatalytic functions of O-GlcNAc transferase. Proceedings of the National Academy of Sciences 2021; 118(4): e2016778118.
[6]. Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Molecular aspects of medicine 2016; 51: 1-15.
[7]. Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. Journal of Biological Chemistry 2012; 287(14): 11070-81.
[8]. Xu X, Peng Q, Jiang X, et al. Altered glycosylation in cancer: molecular functions and therapeutic potential. Cancer Commun (Lond) 2024; 44(11): 1316-36.
[9]. Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-β-D-glucosaminidase silencing on cell phenotype and transcriptome. Journal of Biological Chemistry 2012; 287(34): 28755-69.
[10]. Čaval T, Alisson-Silva F, Schwarz F. Roles of glycosylation at the cancer cell surface: opportunities for large scale glycoproteomics. Theranostics 2023; 13(8): 2605.
[11]. Zhou F, Ma J, Zhu Y, et al. The role and potential mechanism of O-Glycosylation in gastrointestinal tumors. Pharmacological Research 2022; 184: 106420.
[12]. Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. Journal of Biological Chemistry 2014; 289(50): 34457-65.
[13]. Akella NM, Le Minh G, Ciraku L, et al. O-GlcNAc transferase regulates cancer stem–like potential of breast cancer cells. Molecular Cancer Research 2020; 18(4): 585-98.
[14]. Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Science signaling 2013; 6(290): ra75-ra.
[15]. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell metabolism 2017; 25(5): 1027-36.
[16]. Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nature Reviews Cancer 2016; 16(10): 635-49.
Cite this article
Xu,R. (2025). Mechanisms and Applications of OGT-Mediated O-GlcNAc Glycosylation in Malignant Tumors. Theoretical and Natural Science,129,39-48.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhang N, Jiang H, Zhang K, et al. OGT as potential novel target: Structure, function and inhibitors. Chemico-Biological Interactions 2022; 357: 109886.
[2]. Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nature reviews Molecular cell biology 2017; 18(7): 452-65.
[3]. Itkonen HM, Gorad SS, Duveau DY, et al. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget 2016; 7(11): 12464.
[4]. Itkonen HM, Urbanucci A, Martin SE, et al. High OGT activity is essential for MYC-driven proliferation of prostate cancer cells. Theranostics 2019; 9(8): 2183.
[5]. Levine ZG, Potter SC, Joiner CM, et al. Mammalian cell proliferation requires noncatalytic functions of O-GlcNAc transferase. Proceedings of the National Academy of Sciences 2021; 118(4): e2016778118.
[6]. Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Molecular aspects of medicine 2016; 51: 1-15.
[7]. Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. Journal of Biological Chemistry 2012; 287(14): 11070-81.
[8]. Xu X, Peng Q, Jiang X, et al. Altered glycosylation in cancer: molecular functions and therapeutic potential. Cancer Commun (Lond) 2024; 44(11): 1316-36.
[9]. Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-β-D-glucosaminidase silencing on cell phenotype and transcriptome. Journal of Biological Chemistry 2012; 287(34): 28755-69.
[10]. Čaval T, Alisson-Silva F, Schwarz F. Roles of glycosylation at the cancer cell surface: opportunities for large scale glycoproteomics. Theranostics 2023; 13(8): 2605.
[11]. Zhou F, Ma J, Zhu Y, et al. The role and potential mechanism of O-Glycosylation in gastrointestinal tumors. Pharmacological Research 2022; 184: 106420.
[12]. Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. Journal of Biological Chemistry 2014; 289(50): 34457-65.
[13]. Akella NM, Le Minh G, Ciraku L, et al. O-GlcNAc transferase regulates cancer stem–like potential of breast cancer cells. Molecular Cancer Research 2020; 18(4): 585-98.
[14]. Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Science signaling 2013; 6(290): ra75-ra.
[15]. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell metabolism 2017; 25(5): 1027-36.
[16]. Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nature Reviews Cancer 2016; 16(10): 635-49.









