1. Introduction
In vitro nucleic acid amplification, or artificial replication of genetic material, has penetrated into various fields of life science research and has become a key technology in the development of molecular biology. Kary Mullis invented polymerase chain reaction (PCR) in 1983. Through three stages of chain denaturation, primer annealing and enzymatic extension, the artificial in vitro simulation is formed to form a continuous replication of variable temperature amplification system. The amplification system can make the single copy of nucleic acid grow exponentially in a short time, which is convenient for subsequent signal acquisition and analysis. Even though PCR technology is a creative invention, the strong demand for variable temperature systems makes it extremely dependent on expensive thermal cyclers, which largely limits the application of PCR in daily life.
The invention of isothermal amplification technology breaks through the above limitations and provides simplified conditions for the in vitro replication of artificial nucleic acids. The amplification efficiency of isothermal amplification is high, there is no need to repeat the heating and cooling steps in the established program, and multiple molecular reactions can be carried out asynchronously. And the technology does not require expensive variable temperature equipment, which further saves the cost of the experiment. Since the early 1990s, scientists have evolved a variety of isothermal amplification techniques using different principles, The most commonly used are loop-mediated iso-thermal amplification (LAMP) and Recombinase polymerase amplification (Recombinase polymerase amplification, RPA), Cross-priming Amplification (CPA), Strand Displacement Amplification (Strand Displacement Amplification) SDA), Rolling Circle Amplification (RCA), helicase-dependent amplification (HAD).
In the PubMed database, the results of ‘loop mediated isothermal amplification OR LAMP’ as the keyword were 965. ‘Loop mediated isothermal amplification’ in Web of Science, whose result is as high as 9835. The data show that the published papers in this field increase year by year from 2007 to 2023, indicating that the innovation of this technology is still the hot spot in the field. During the review process, it was found that most of the literature was about the application of LAMP technology in molecular detection, covering the detection of bacteria, viruses and parasites in many industries such as food safety, medical inspection, planting, animal husbandry, and aquatic products, indicating that LAMP technology has a wide range of applications and expansibility.
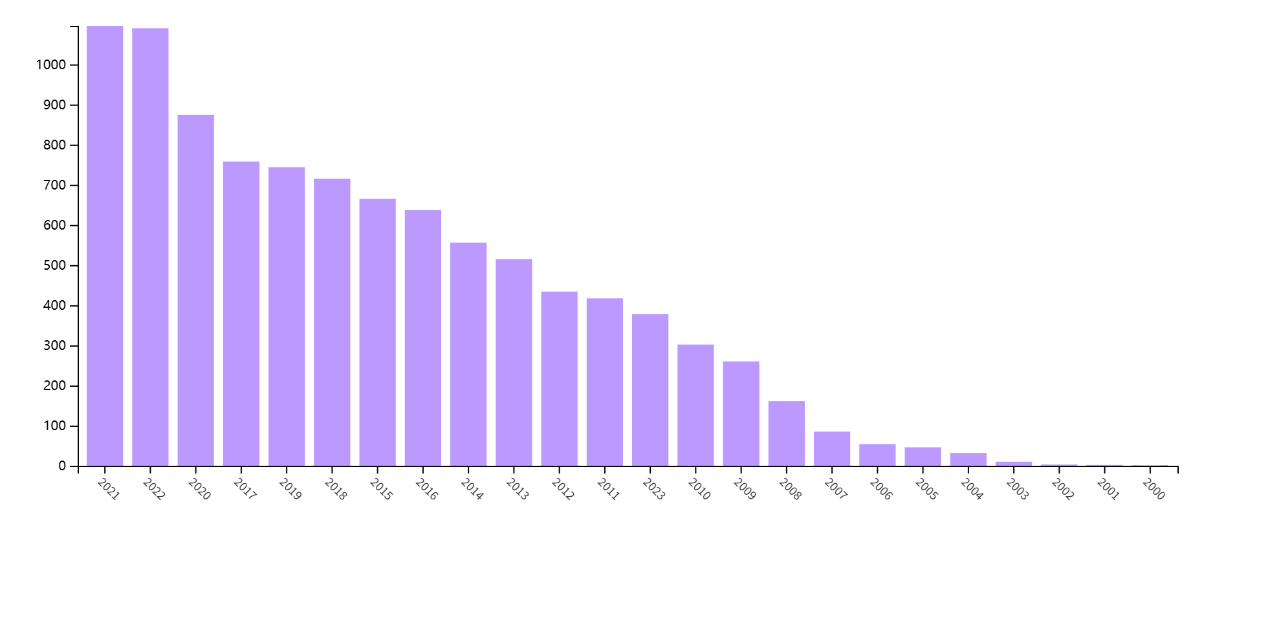
Figure 1. Annual publication volume of related literature on Web of Science.
2. LAMP technology
2.1. Advantages of LAMP
1. LAMP technology does not need high and low temperature cycle, can achieve isothermal amplification (60℃~65℃), so the equipment requirements are low, only water bath pan or thermos bottle is needed
2. The amplification efficiency is high, and the exponential growth of several copies to 109 copies can be achieved within 60min
3. High sensitivity, the detection limit can reach 1.4×10-1 pg DNA
4. Strong specificity, 4 primers identify 6 sites of the target gene, any region does not match the primer can not be nucleic acid amplification, to ensure the specificity of amplification
5. Low cost, no need for accurate temperature control system and special reagents, suitable for clinical rapid detection
6. The template can be crude DNA, and the test results can be interpreted by the naked eye
2.2. Disadvantages of LAMP
1. The design requirements of primers are high, and all primers need to match for amplification
2. The amplified fragment is too small, and the length of the target sequence must be controlled below 300bp
3. The amplified products can not be used for cloning sequencing, only for judgment, and the reaction results can not be used for quantitative analysis
4. Nucleotide sites with single base differences are of poor applicability
5. It is easy to form aerosols and cause false positives
6. It is difficult to design internal and external comparison of reaction system
3. New LAMP technology for COVID-19 detection
While LAMP is used to detect various pathogens, it is also gradually used for qualitative and quantitative detection of viruses, bacteria and parasites. This technology is simpler and more convenient than PCR technology both in terms of practical operation and instrument requirements [1]. In the 2019 novel coronavirus outbreak, LMAP has been successfully used to develop a rapid SARS-CoV-2 nucleic acid test kit for home use, which only takes 20 minutes to achieve rapid detection of the novel coronavirus [2]. In addition, some new technologies based on LAMP are also showing off in the novel coronavirus test.
3.1. RT-LAMP technology
RT-LAMP is reverse transcription-ring mediated isothermal amplification, which is generally considered to be between RT-qPCR and rapid antigen detection in terms of sensitivity and simplicity. RT-LAMP was found to be effective in detecting viruses in RT-qPCR amplified samples that were amplified under a quantitative cycle (Cq) of < 30. For samples with Cq values in the range of 32 to 35, the reliability of RT-LAMP was reduced [3-4].
Because the sample is RNA, which is easily degraded by RNA enzymes during extraction and amplification, higher requirements are put forward for the reverse transcriptional LAMP system. In the most direct form of detection, as the reaction heats up, viral particles release the RNA into the solution, exposing them to RNase. The RT enzyme must synthesize the first cDNA strand before the RNase degrades the viral RNA, so it can be described as a race between the RT enzyme and the RNase [5]. Work by Sun et al. using digital RT-LAMP shows that the RT step is significantly less than 100% efficient, so multiple RNA copies must survive to trigger exponential amplification and positive results [6]. It was found that the RNase can be saturated by adding large amounts of tRNA [7], or the RNase inhibitor can be incorporated to suppress the RNase activity in a stoichiometric manner, but the reagent price can be very expensive [8]. Pretreatment before mixing with the premix can effectively improve the above problems. Usually, RNA is treated with a strong alkaline solution, and the treated sample is diluted in the RT-LAMP premix, so that the pH is about 8.5~9.0[9]. Enzyme pretreatment with protease K is another technique to achieve RNase inactivation. Polymerase and RNase can be digested under high temperature and denaturation conditions, so inactivation is required before adding RT-LAMP enzyme. Thermally unstable protease K becomes an alternative to inactivation near the RT-LAMP reaction temperature. Lalli et al. demonstrated that regimens containing protease K had better performance [10]. Full extraction and purification of RT-LAMP was achieved by Rabe and Cepko using a bulk silica matrix [11]. Bokelmann et al. selectively enriched SARS-CoV-2 RNA using oligonucleotide-coupled magnetic beads, and purified the RNA using low-salt wash heating elution [12]. Kondo et al. also used antiboy-coupled magnetic beads to concentrate and purify relevant RNA [13].
Yang et al. applied RT-LAMP to the rapid detection of SARS-CoV-2, using different primers target the Orf1ab gene, S gene and N gene, and the detection limit was 80 copies per ml [14]. It has also been suggested that not all targets in the Orf1a and N genes have the same sensitivity [15]. During the COVID-19 pandemic, many teams simultaneously conducted RT-LAMP research for SARS-CoV-2, and the principle and conclusion were roughly the same [16-20].
3.2. LAMP-CRISPR technology
LAMP-based CRISPR technology is a new generation of molecular detection technology developed in recent years, through the combination of the two can effectively eliminate the limitation of their single operation.
In the selection of Cas enzyme, Cas12 endonuclease is preferred because of its collateral cleavage activity. Among them, Cas12a and Cas12b are most commonly used for LAMP-CRISPR detection [21]. The target of Cas13 is single-stranded RNA, and it is worth noting that since Cas13a protein is only triggered by the RNA target, an additional T7 transcription step is required to convert the DNA amplification into RNA after the RT-LAMP reaction [22-23].
The LAMP-CRISPR technology has shown high specificity and sensitivity in pathogen detection. Compared with qPCR, the LAMP-CRISPR test has a shorter turnaround time, making it suitable for rapid detection. However, LAMP-CRISPR still has some limitations. The amplification and CRISPR processes require different reagents and reaction conditions: the Bst enzyme used in amplification reacts at 60-65 ° C, while the Cas enzyme used in CRISPR reacts at around 37°C, so using two different reaction systems may increase the risk of contamination.
Broughton et al. developed a fast (≤12 minutes, easy to implement, and accurate CRISPR-Cas12-based side-flow assay for the detection of SARS-CoV-36 in respiratory swab RNA extracts [24]. Zhang et al report an RT-LAMP-CRISPR assay based on Cas12a for the detection of SARS-CoV-2 nsp8 and N genes [25]. Chandrasekaran et al. developed a DISCoVER technique involving dissolution of extraction-free samples by low-cost reagents, multiple isothermal RNA amplification and T7 transcription, and Cas13-mediated cleavage of quenching fluorophors with a sensitivity of 40 copies of µL-1[26]. Verma et al. reviewed the mechanisms of action of different enzymes based on Cas9, Cas12, and Cas13, and elucidated the advantages of selection and improved methods [27].
3.3. LAMP-LFD
Lateral flow detect (Lateral flow detect) is a new method for detecting LAMP amplification products, It includes 3 steps: nucleic acid amplification (LAMP), molecular hybridization (hybridization of biotin-labeled LAMP amplification product with fluorescein labeled probe) and lateral flow detection (binding with fluorescein antibody, biotin antibody and fluorescein antibody labeled on the test strip through lateral chromatography to show specific colors on the detection line and quality control line). At present, LAMP-LFD technology has been used in various biological detection fields, and has the advantages of high sensitivity, high specificity, fast, convenient, low cost and easy product detection [28]. This technology has been applied to the detection of novel coronavirus. Simon et al. combined RT-LAMP with lateral flow technology (LFD), which can detect two gene amplification on a single strip, and is suitable for multiple detection. Chen et al. used a nanoparticle based side-flow biosensor to analyze mRT-LAMP products, and optimized conditions such as target RNA concentration, amplification temperature and time in MRT-LAMP-LFB amplification. In this study, mRT-LAMP-LFB assay was applied to detect SARS-CoV-2 virus from clinical samples and artificial sputum samples [29].
3.4. LAMP-Chip
In order to meet the needs of POC molecular diagnosis, Byung et al. proposed an integrated rotary microfluidic system. The chip is composed of three identical units, each of which consists of solid phase DNA extraction, LAMP reaction and lateral flow strip [30]. Fang et al. designed eight parallel reaction channels on the chip, each requiring only 0.4μL of sample, and a single channel combined with a photoconductive fiber can achieve a detection limit of 10 fg/μL [31]. Microfluidic chip technology can integrate sample processing, product detection and result output into one, so as to better adapt to the needs of field detection.
For COVID-19 detection, Torezin et al. developed an instant lab-on-a-chip instrument to diagnose SARS-CoV-2 using RT-LAMP. The microfluidics chip was kept at ~ (65.0±0.1) ℃ for 25 minutes and cooled for 5 minutes [32]. Colbert et al. combined RT-LAMP with particle imaging technology particle diffusion polarization to successfully detect SARS-CoV-30 on a portable chip with integrated heating in only 35 minutes, detecting as few as 2 virus particles per μL, and imaging a sample containing fluorescent beads using a smartphone device [33]. Song et al. proposed a fast MUSAL chip, powered by a simple LED for photothermal amplification operation and can detect 500 targets from a single swab sample with pollution-free amplification [34]. Rodriguez-Mateos et al. designed an integrated chip platform that combines surface tension-assisted immiscible filtration (IFAST) with RNA amplification and detection to provide a viable means of screening for SARS-CoV-2 infection in resource-limited settings [35].
4. Conclusion
As one of the widely used thermostatic amplification technologies, LAMP technology is convenient, immediate, easy to operate, strong specificity, etc. Compared with gold standard PCR, it is more suitable for clinical diagnosis or field detection in public health environment. However, although LAMP can solve the temperature control problem of PCR well, it also brings other problems correspondingly, such as insufficient detection sensitivity, high difficulty in primer design, easy to cause contamination and false positives, and can not be accurately quantified. With the continuous improvement of technology and integration with new technologies, the shortcomings of LAMP have been corrected, such as DETECTR and SHERLOCK technology based on Cas enzyme, and immunoassay platform for pH change through engineering and optimized isothermal nucleic acid amplification. The future trend will be combined with microfluidic chip and computer program assistance to integrate nucleic acid amplification and result detection in a chip, to achieve integration of amplification and detection, automation, portability, so that LAMP technology becomes a supplement and replacement of PCR.
In the face of public health emergencies similar to COVID 19, we can also make LAMP technology into a rapid test kit, using its advantages of constant temperature detection to efficiently screen the outbreak point, so as to more effectively control the spread of the epidemic. This kind of testing method is also applicable to poor areas with poor medical conditions.
References
[1]. Kashir J, Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med Hypotheses. 2020; 141:109786.
[2]. Liu Wang, Jin Jinghao, Chen Xiaoren. Advances in loop-mediated isothermal amplification [J]. Advances in Biotechnology, 2019,11(02):128-135.
[3]. DaoThi VL, Herbst K, Boerner K, et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020; 12(556):eabc7075.
[4]. Amaral C, Antunes W, Moe E, et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci Rep. 2021; 11 (1) : 1-12.
[5]. Choi G, Moehling TJ, Meagher RJ. Advances in RT-LAMP for COVID-19 testing and diagnosis. Expert Rev Mol Diagn. 2023; (1) : 23 September 28.
[6]. Sun B, Shen F, McCalla SE, et al. Mechanistic evaluation of the pros and cons of digital RT-LAMP for HIV-1 viral load quantification on a microfluidic device and improved efficiency via a two-step digital protocol. Anal Chem. 2013; 85 (3) : 1540-1546.
[7]. Wei S, Suryawanshi H, Djandji A, et al. Field-deployable, rapid diagnostic testing of saliva for SARS-CoV-2. Sci Rep. 2021; 11 (1) : 5448.
[8]. Janikova M, Hodosy J, Boor P, et al. Loop-mediated isothermal amplification for the detection of SARS-CoV-2 in saliva. Microb Biotechnol. 2021; 14 (1) : 307-316.
[9]. Bender AT, Sullivan BP, Lillis L, et al. Enzymatic and Chemical-Based Methods to Inactivate Endogenous Blood Ribonucleases for Nucleic Acid Diagnostics. J Mol Diagn. 2020; 22 (8) : 1030-1040.
[10]. Lalli MA, Langmade JS, Chen X, et al. Rapid and extraction-free detection of SARS-CoV-2 from saliva by colorimetric reverse-transcription loop-mediated isothermal amplification. Clin Chem. 2021; 67 (2) : 415-424.
[11]. Rabe BA, Cepko C. SARS-CoV-2 detection using isothermal amplification and a rapid, inexpensive protocol for sample inactivation and purification. Proc Nat Acad Sci. 2020; 117 (39) : 24450-24458.
[12]. Bokelmann L, Nickel O, Maricic T, et al. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat Commun. 2021; 12 (1) : 1467.
[13]. Kondo T, Iwatani Y, Matsuoka K, et al. Antibody-like proteins that capture and neutralize SARS-CoV-2. Sci Adv. 2020; 6(42):eabd3916.
[14]. Huang WE, Lim B, Hsu CC, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020; 13 (4) : 950-961.
[15]. Aldossary AM, Tawfik EA, Altammami MA, et al. Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics (Basel). 2022; 12 (9) : 2232.
[16]. Chow FW, Chan TT, Tam AR, et al. A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19. Int J Mol Sci. 2020; 21 (15) : 5380.
[17]. Dao Thi VL, Herbst K, Boerner K, et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020; 12(556):eabc7075.
[18]. Sherrill-Mix S, Hwang Y, Roche AM, et al. Detection of SARS-CoV-2 RNA using RT-LAMP and molecular beacons. Genome Biol. 2021; 22 (1) : 169.
[19]. Zhang Y, Chen M, Liu C, et al. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens Actuators B Chem. 2021; 345:130411.
[20]. Nandi SS, Lambe UP, Sawant SA, et al. Development of a RT-LAMP assay for detection of SARS-CoV-2. Indian J Med Res. 2022; 155 (1) : 148-155.
[21]. Teng F, Cui T, Feng G, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018; 3.
[22]. Joung J, Ladha A, Saito M, et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. Preprint. medRxiv. 2020; 2020.05.04.20091231.
[23]. Ali Z, Aman R, Mahas A, et al. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020; 288:198129.
[24]. Broughton JP, Deng X, Yu G, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020; 38 (7) : 870-874.
[25]. Zhang Y, Chen M, Liu C, et al. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens Actuators B Chem. 2021; 345:130411.
[26]. Chandrasekaran SS, Agrawal S, Fanton A, et al. Rapid detection of SARS-CoV-2 RNA in saliva via Cas13. Nat Biomed Eng. 2022; 6 (8) : 944-956.
[27]. Verma MK, Roychowdhury S, Sahu BD, et al. CRISPR-based point-of-care diagnostics incorporating Cas9, Cas12, and Cas13 enzymes advanced for SARS-CoV-2 detection. J Biochem Mol Toxicol. 2022; 36(8):e23113.
[28]. Huang Hai-Long, Zhu Peng, Yang Hao. LAMP-LFD technology and its application in bioassay [J]. Chinese Journal of Bioengineering,2015,35(12):89-95.
[29]. Chen X, Zhou Q, Li S, et al. Rapid and Visual Detection of SARS-CoV-2 Using Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Linked With Gold Nanoparticle-Based Lateral Flow Biosensor. Front Cell Infect Microbiol. 2021; 11:58. 1239.
[30]. Park BH, Oh SJ, Jung JH, et al. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens Bioelectron. 2017; 91:334-340.
[31]. Fang X, Liu Y, Kong J, et al. Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem. 2010; 82 (7) : 3002-6.
[32]. Torezin Mendonca G, Cassaboni Stracke M, de Oliveira Coelho B, et al. A new RT-LAMP-on-a-Chip Instrument for SARS-CoV-2 diagnostics. Microchem J. 2022; 180:107600.
[33]. Colbert AJ, Lee DH, Clayton KN, et al. PD-LAMP smartphone detection of SARS-CoV-2 on chip. Anal Chim Acta. 2022; 1203:339702.
[34]. Song M, Hong S, Lee LP. Multiplexed Ultrasensitive Sample-to-Answer RT-LAMP Chip for the Identification of SARS-CoV-2 and Influenza Viruses. Adv Mater. 2023; 35(10):e2207138.
[35]. Rodriguez-Mateos P, Ngamsom B, Walter C, et al. A lab-on-a-chip platform for integrated extraction and detection of SARS-CoV-2 RNA in resource-limited settings. Anal Chim Acta. 2021; 1177:338758.
Cite this article
Wang,Y. (2023). Loop-mediated Iso-thermal amplification (LAMP) used for COVID-19 detection. Theoretical and Natural Science,8,96-102.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kashir J, Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med Hypotheses. 2020; 141:109786.
[2]. Liu Wang, Jin Jinghao, Chen Xiaoren. Advances in loop-mediated isothermal amplification [J]. Advances in Biotechnology, 2019,11(02):128-135.
[3]. DaoThi VL, Herbst K, Boerner K, et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020; 12(556):eabc7075.
[4]. Amaral C, Antunes W, Moe E, et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci Rep. 2021; 11 (1) : 1-12.
[5]. Choi G, Moehling TJ, Meagher RJ. Advances in RT-LAMP for COVID-19 testing and diagnosis. Expert Rev Mol Diagn. 2023; (1) : 23 September 28.
[6]. Sun B, Shen F, McCalla SE, et al. Mechanistic evaluation of the pros and cons of digital RT-LAMP for HIV-1 viral load quantification on a microfluidic device and improved efficiency via a two-step digital protocol. Anal Chem. 2013; 85 (3) : 1540-1546.
[7]. Wei S, Suryawanshi H, Djandji A, et al. Field-deployable, rapid diagnostic testing of saliva for SARS-CoV-2. Sci Rep. 2021; 11 (1) : 5448.
[8]. Janikova M, Hodosy J, Boor P, et al. Loop-mediated isothermal amplification for the detection of SARS-CoV-2 in saliva. Microb Biotechnol. 2021; 14 (1) : 307-316.
[9]. Bender AT, Sullivan BP, Lillis L, et al. Enzymatic and Chemical-Based Methods to Inactivate Endogenous Blood Ribonucleases for Nucleic Acid Diagnostics. J Mol Diagn. 2020; 22 (8) : 1030-1040.
[10]. Lalli MA, Langmade JS, Chen X, et al. Rapid and extraction-free detection of SARS-CoV-2 from saliva by colorimetric reverse-transcription loop-mediated isothermal amplification. Clin Chem. 2021; 67 (2) : 415-424.
[11]. Rabe BA, Cepko C. SARS-CoV-2 detection using isothermal amplification and a rapid, inexpensive protocol for sample inactivation and purification. Proc Nat Acad Sci. 2020; 117 (39) : 24450-24458.
[12]. Bokelmann L, Nickel O, Maricic T, et al. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat Commun. 2021; 12 (1) : 1467.
[13]. Kondo T, Iwatani Y, Matsuoka K, et al. Antibody-like proteins that capture and neutralize SARS-CoV-2. Sci Adv. 2020; 6(42):eabd3916.
[14]. Huang WE, Lim B, Hsu CC, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb Biotechnol. 2020; 13 (4) : 950-961.
[15]. Aldossary AM, Tawfik EA, Altammami MA, et al. Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics (Basel). 2022; 12 (9) : 2232.
[16]. Chow FW, Chan TT, Tam AR, et al. A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19. Int J Mol Sci. 2020; 21 (15) : 5380.
[17]. Dao Thi VL, Herbst K, Boerner K, et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020; 12(556):eabc7075.
[18]. Sherrill-Mix S, Hwang Y, Roche AM, et al. Detection of SARS-CoV-2 RNA using RT-LAMP and molecular beacons. Genome Biol. 2021; 22 (1) : 169.
[19]. Zhang Y, Chen M, Liu C, et al. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens Actuators B Chem. 2021; 345:130411.
[20]. Nandi SS, Lambe UP, Sawant SA, et al. Development of a RT-LAMP assay for detection of SARS-CoV-2. Indian J Med Res. 2022; 155 (1) : 148-155.
[21]. Teng F, Cui T, Feng G, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018; 3.
[22]. Joung J, Ladha A, Saito M, et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. Preprint. medRxiv. 2020; 2020.05.04.20091231.
[23]. Ali Z, Aman R, Mahas A, et al. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020; 288:198129.
[24]. Broughton JP, Deng X, Yu G, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020; 38 (7) : 870-874.
[25]. Zhang Y, Chen M, Liu C, et al. Sensitive and rapid on-site detection of SARS-CoV-2 using a gold nanoparticle-based high-throughput platform coupled with CRISPR/Cas12-assisted RT-LAMP. Sens Actuators B Chem. 2021; 345:130411.
[26]. Chandrasekaran SS, Agrawal S, Fanton A, et al. Rapid detection of SARS-CoV-2 RNA in saliva via Cas13. Nat Biomed Eng. 2022; 6 (8) : 944-956.
[27]. Verma MK, Roychowdhury S, Sahu BD, et al. CRISPR-based point-of-care diagnostics incorporating Cas9, Cas12, and Cas13 enzymes advanced for SARS-CoV-2 detection. J Biochem Mol Toxicol. 2022; 36(8):e23113.
[28]. Huang Hai-Long, Zhu Peng, Yang Hao. LAMP-LFD technology and its application in bioassay [J]. Chinese Journal of Bioengineering,2015,35(12):89-95.
[29]. Chen X, Zhou Q, Li S, et al. Rapid and Visual Detection of SARS-CoV-2 Using Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Linked With Gold Nanoparticle-Based Lateral Flow Biosensor. Front Cell Infect Microbiol. 2021; 11:58. 1239.
[30]. Park BH, Oh SJ, Jung JH, et al. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens Bioelectron. 2017; 91:334-340.
[31]. Fang X, Liu Y, Kong J, et al. Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem. 2010; 82 (7) : 3002-6.
[32]. Torezin Mendonca G, Cassaboni Stracke M, de Oliveira Coelho B, et al. A new RT-LAMP-on-a-Chip Instrument for SARS-CoV-2 diagnostics. Microchem J. 2022; 180:107600.
[33]. Colbert AJ, Lee DH, Clayton KN, et al. PD-LAMP smartphone detection of SARS-CoV-2 on chip. Anal Chim Acta. 2022; 1203:339702.
[34]. Song M, Hong S, Lee LP. Multiplexed Ultrasensitive Sample-to-Answer RT-LAMP Chip for the Identification of SARS-CoV-2 and Influenza Viruses. Adv Mater. 2023; 35(10):e2207138.
[35]. Rodriguez-Mateos P, Ngamsom B, Walter C, et al. A lab-on-a-chip platform for integrated extraction and detection of SARS-CoV-2 RNA in resource-limited settings. Anal Chim Acta. 2021; 1177:338758.









