1. Introduction
Osteoarthritis (OA) is a chronic musculoskeletal disorder characterized by joint structural alterations (e.g., osteophyte formation, cartilage degeneration, and bone remodeling) [1]. In the early period, the disorder has many negative effects for individuals with OA, such as pain, brief morning stiffness, and functional limitations. Further, the osteoarthritic process leads to disability. The Global Burden of Disease (GBD) Study 2019 found that the individuals with OA-related disability increased by 114.5% between 1990 and 2019, from 247.51 million to 527 .81 million [2]. Recently, the prevalence of OA is exacerbated by increases of aging population and obesity globally. It has pointed out that age is among the most evident risk factors associated with OA. For example, the global age-standardized prevalence rate of OA continues to increase from age 30 to 64, and then slowly begins to decline at age 64. In all age groups, female patients with OA still were higher than male patients [2]. In addition, obesity constitutes a significant risk factor linked to OA. A study found the rate of adults with obese (Body Mass Index [BMI] ≥ 35) increases from 21.26% in 2006 to 26% in 2019[3]. In fact, Obese people are twice as likely as individuals with a BMI below 25 to develop symptomatic OA during their lifetime [4].
OA prevalence is changing with the joints, mainly including knee, hip and hand. The GBD study 2019 found that the population of individuals reporting KOA was the highest, with 60.6%. The hand osteoarthritis (OA) had a prevalence rate of 23.7%, while the hip OA had a prevalence rate of 5.5% [2]. Recent research found that the age of patients with KOA is becoming younger and the majority of the young patients have suffered different types of knee injuries [5]. The early prevalence in young population may expands the burden of OA worldwide. In terms of direct impacts, this trend may increase the medical costs, such as knee joint replacements. In terms of indirect impacts, these youths with KOA encounter unemployment, diminished quality of life, decreased activity, and depression. As the rise of aging and obesity, the situation will get worse. Hence, it is essential to understand some risks factors and preventions for KOA among young adults. This paper aims to review risk factors and non-pharmacologic treatments for OA among young adults.
2. Methods
2.1. Data sources
The Global Burden of Disease (GBD) Study 2019 was published by the Institute for Health Metrics and Evaluation (IHME) in 2020. The GBD Study 2019 assesses the burden and epidemiology of 369 diseases and injuries in 204 countries and territories from 1990 to 2019. This article uses data from this report, including: the number and the prevalence of individuals with knee osteoarthritis (KOA) by sex and age groups in total of 204 countries and territories from 1990 to 2019 (https://vizhub.healthdata.org/gbd-results/). In the GBD study 2019, OA was defined as symptomatic OA with Kellgren/Lawrence grades 2-4 that were radiologically confirmed and pain that had persisted for a minimum of one month within the previous twelve months.
2.2. Statistical analysis
This article presents a comparative analysis of the number and the prevalence of individuals with KOA between 2019 and 2019. The comparison is conducted based on gender, encompassing both males and females, as well as age categories, which consist of 20 distinct cohorts. The analysis software utilized in this paper is EXCELL from Microsoft Office (Home and Student Edition 2021). This software is responsible for generating Table 1, Figure 1, and Figure 2.
3. Prevalence and incidence
According to the GBD Study 2019, the number of individuals with KOA have been accounted for approximately 364.58 million (95% UI 315.25-417.40), while the number of individuals with hand OA and hip OA were 142.48 million (95% UI 108.63-186.79) and 32.99 million (95% UI 25.69-41.12) in 2019. The age-standardized prevalence rate (ASR) of KOA was 4.38 ×103 per 100,000 (95% UI 3.79, 5.00). Although the people aged 45 years and older with KOA is account for the largest number, it is crucial that the young adults aged 30-44 years with KOA may suffer from negative consequence for longer [2]. As shown in Figure 1, the number of young individuals aged 30-34 (6.92 million), 35-39 (58.82 million) and 40-44 (157.58 million) suffering from KOA is increasing successively. In addition, the number of female patients is greater than that of male patients across all age categories, and this disparity is growing. For the three age groups listed above, the prevalence of KOA increased with age, from 0.12% (95%UI 0.09%-0.16%) in the age group of 30-34 to 3.22% (95%UI 2.45%-4.02%) in the age group of 40-44. Females between the ages of 40 and 44 had the greatest prevalence, with 3.78% (95% UI 2.88%-4.74%). This was followed by men aged 40-44 years and whose prevalence was 2.67% (95% UI 2.01%- 3.37%). For individual perspective, OA is the principal cause of pain and mobility impairment. As individuals with osteoarthritis aged, they became less active, which may affect health outcomes (weight gain, co-morbidities) and increase the risk of joint arthroplasty [6]. In the context of quality of life (QOL), it has been observed that adolescent athletes who have had injuries tend to report a decline in the overall quality of social networks and interpersonal connections, including notable shifts in mental well-being and lifestyle patterns, in comparison to their uninjured peers.
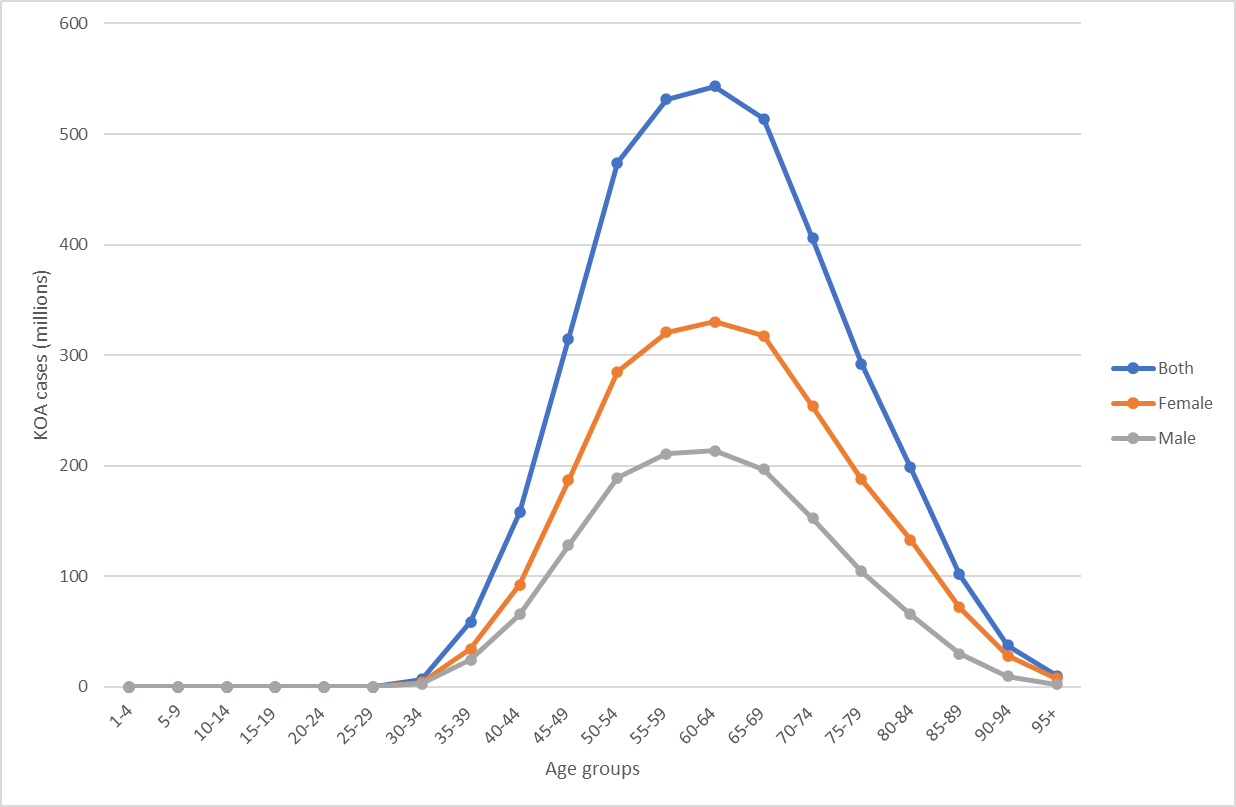
Figure 1. The number of individuals with knee osteoarthritis by sex and age groups.
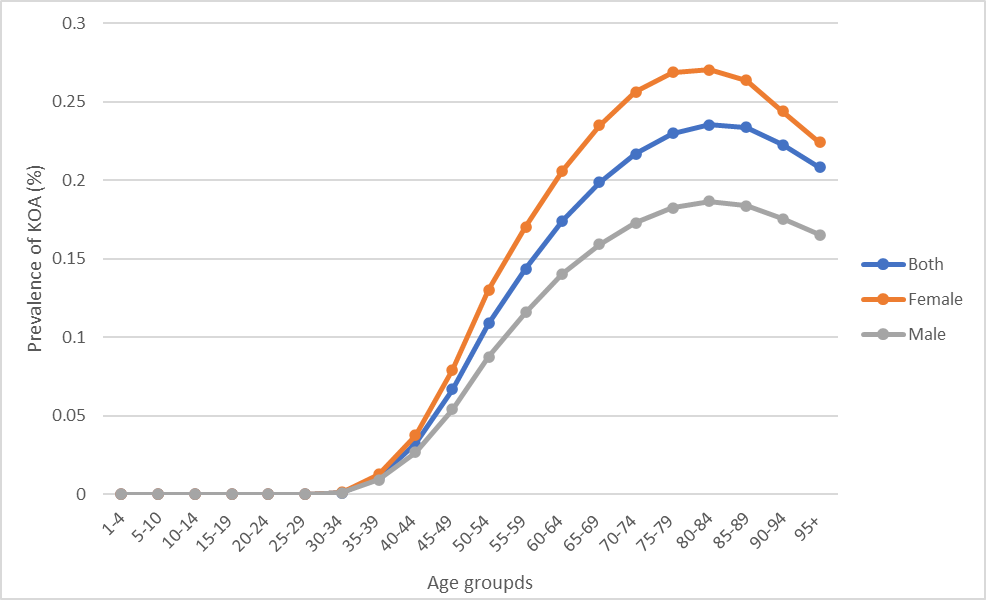
Figure 2. The prevalence of individuals with knee osteoarthritis by sex and age groups.
Table 1. Number of individuals (millions) with knee osteoarthritis and prevalence (%) for in 2019.
Age range | Number of individuals with knee osteoarthritis (millions) | Prevalence (%) | ||||
Both | Female | Male | Both | Female | Male | |
1-4 | 0 | 0 | 0 | 0 | 0 | 0 |
5-10 | 0 | 0 | 0 | 0 | 0 | 0 |
10-14 | 0 | 0 | 0 | 0 | 0 | 0 |
15-19 | 0 | 0 | 0 | 0 | 0 | 0 |
20-24 | 0 | 0 | 0 | 0 | 0 | 0 |
25-29 | 0 | 0 | 0 | 0 | 0 | 0 |
30-34 | 6.92 (5.04,9.21) | 4.03 (2.94,5.37) | 2.88 (2.09,3.85) | 0.12 (0.09,0.16) | 0.14( 0.10,0.18) | 0.10 (0.07,0.13) |
35-39 | 58.82 (42.82,77.79) | 34.25 (25.08,45.19) | 24.57 (17.79,32.63) | 1.10 (0.80,1.45) | 1.28 (0.94,1.69) | 0.92 (0.66,1.22) |
40-44 | 157.58 (119.6,198.59) | 92.23 (70.39,115.77) | 65.35 (49.21,82.69) | 3.22 (2.45,4.05) | 3.78 (2.88,4.74) | 2.67 (2.01,3.37) |
45-49 | 314.64 (249.44,379.74) | 186.55 (148.15,225.01) | 128.09 (101.28,154.89) | 6.68 (5.31,8.07) | 7.94( 6.31,9.57) | 5.43 (4.31,6.57) |
50-54 | 473.92 (365.4,587.26) | 284.78 (219.73,351.87) | 189.14 (145.31,235.28) | 10.90 (8.40,13.49) | 13.01 (10.04,16.08) | 8.76 (6.73,10.89) |
55-59 | 531.3 (431.64,636.05) | 320.43 (261.33,382.88) | 210.87 (170.99,253.58) | 14.36 (11.67,17.19) | 17.05 (13.90,20.37) | 11.59 (9.39,13.93) |
60-64 | 543.27 (439.8,654.15) | 330.04 (267.83,395.86) | 213.23 (171.7,258.02) | 17.41 (14.10,20.97) | 20.60 (16.71,24.71) | 14.05 (11.32,17.00) |
65-69 | 513.72 (423.61,608.41) | 317.24 (262.99,374.73) | 196.47 (160.48,233.43) | 19.88 (16.40,23.55) | 23.52 (19.49,27.78) | 15.91 (12.99,18.90) |
70-74 | 405.81 (338.66,475.34) | 253.65 (211.96,296.05) | 152.15 (125.61,180.09) | 21.70 (18.11,25.42) | 25.63 (21.42,29.92) | 17.28 (14.27,20.45) |
75-79 | 292.07 (248.24,339.94) | 187.73( 159.95,216.99) | 104.34 (88.26,122.38) | 22.99 (19.54,26.76) | 26.88 (22.90,31.07) | 18.25 (15.43,21.40) |
80-84 | 198.67 (167.67,233.15) | 132.96 (112.37,154.75) | 65.71 (55.22,77.97) | 23.54 (19.86,27.62) | 27.04 (22.85,31.47) | 18.65 (15.68,22.13) |
85-89 | 101.61 (85.58,119.39) | 71.67 (60.63,84.15) | 29.94 (25.03,35.69) | 23.37 (19.68,27.46) | 26.35 (22.30,30.94) | 18.39 (15.37,21.92) |
90-94 | 37.5 (31.59,44.51) | 28.19 (23.81,33.33) | 9.31 (7.78,11.21) | 22.25 (18.74,26.41) | 24.41 (20.61,28.86) | 17.55 (14.67,21.11) |
95+ | 9.94 (8.33,11.76) | 7.83 (6.57,9.28) | 2.11 (1.76,2.53) | 20.83 (17.45,24.65) | 22.41 (18.79,26.54) | 16.53 (13.82,19.81) |
4. Risk factors for young adults with KOA
4.1. Age
A study has revealed that KOA risk increases with age among the people between the ages of 40 and 65 with osteoarthritis, which is similar with the relationship between overall individuals with KOA and age [2]. However, a population-based cohort study found that people under 30 years old were more likely to have KOA than people over 30 years old [7]. The tendency of KOA patients to be younger can be attributed to the excessive amounts of sports and exercise that young people participate in. The more time youthful and athletic individuals spend participating in professional and recreational activities, the greater their risk of injury, which can increase their likelihood of developing osteoarthritis. Further, professional runners were more likely to have KOA than recreational runners, but not more likely than individuals who didn’t run. In addition to the type of physical activity, adolescent individuals involved in sporting activities characterized by contact, rapid alterations in direction, and sudden instances of acceleration or deceleration, are more susceptible to injuries [1]. In one study, individuals in football, weightlifting and wrestling were more likely to have KOA in contrast to those who did not engage in these sport activities [5].
4.2. Sex
Some epidemiological studies have shown that the incidence of KOA among young female is higher compare with young male [2]. The observed phenomena could potentially be attributed to anatomical differences in the knee joint among males and females. Compared with males’ knee anatomy, females have narrower femurs, thinner patellae and slightly bigger quadriceps angle and different size between lateral and medial tibial condyles [8]. This difference can have an influence on the knee kinematics for women, which can contribute to the occurrence of knee injuries and eventually result in progression of KOA. For example, in a single-leg landing experiment on healthy males and females, the female lower limbs showed greater knee valgus excursion (females: 7.10 CI: 5.78-8.41 vs. males: 4.39 (95% CI: 3.69-85.10), p=0.01) and knee varus excursion (females: 5.57 CI: 4.01-7.10 vs. males: 3.42 (95% CI: 2.06-4.77), p=0.03), and bigger knee varus velocity (approximately 2.7 deg/sec more, p=0.04) and knee valgus velocity (approximately 2.15 deg/sec more, p=0.03), which may contribute a potential risk to knee injuries for females [9].
4.3. Obesity
Youth who are obese have an increased risk of developing KOA. For example, the research shows that there is a higher association between KOA and young females (aged ≤ 45 years old) with obesity compared with the matched group who have no obesity, about 2.5-fold [10]. From a mechanical perspective, the buildup of excessive or abnormally fat can result in an increased stress on the major principal load-bearing joints, particularly hip and knee. Moreover, the additional burden might lead to an acceleration in cartilage degradation and make the cartilage more prone to disruption caused by physical forces. At the same time, obesity can also lead to OA in non-heavy joints. From a metabolic and inflammatory perspective, the products secreted by adipose tissue and the physiological integrity of adipose tissue may serves as the fundamental framework for the synthesis of pro-inflammatory molecules, which can initiate the development of KOA [8]. Leptin, a hormone synthesized by adipose tissue, has a significant role in the regulation of both obesity and osteoarthritis. Leptin facilitates the production of pro-inflammatory cytokines, specifically IL-6, by synovial fibroblasts [11]. Fortunately, weight lost is a practical way for patients with KOA. A study shows that weight reduction has a positive effect on knee pain, and the effect of weight loss treatment combined with appropriate physical exercise is better than that of physical exercise or weight loss treatment alone [1].
4.4. Genetics
Genetics plays an important role to OA susceptibility. Recently, there is a notable increase in the identification and reporting of OA risk loci. For example, over 90 OA risk loci have been found in the genome-wide association studies (GWAS) [8]. Furthermore, Certain writers argue that changes in genetic vulnerability to OA mostly stem from modifications in the control of gene expression, rather than modifications in the protein sequence [12].
4.5. Joint Trauma
A population-based cohort study has shown that previous knee trauma is the greatest predictor of the development of KOA in juvenile and middle-aged adults aged 25-34 years. It found that individuals with cruciate ligament, meniscal injury, or intra-articular fracture had a 7.6-fold (95% CI: 5.9-11.4), 8.2-fold (95% CI: 5.5-10.5), and 7.0-fold (95% CI: 4.2 -11.7) to develop KOA, respectively, compared to the healthy population without knee trauma [7]. At the same time, the combination of different kinds of prior traumatic joint injury and subsequent surgery is highly related to the risk of KOA for the young. The research demonstrates that individuals who only experienced meniscal tear, those who only experienced cruciate ligament tear, and those who experienced both the two injuries were 7.3 times (95% CI: 5.4-9.9), 8.4 times (95%CI: 6.3-11.1) and 9.0 times (95%CI: 5.8-14.0) to develop KOA than the normal population without knee trauma [7]. OA is a while joint disease, which mainly effects the normal metabolic activity. These different traumatic joint injuries exacerbate the anabolic-catabolic activity imbalance in the affected joints.
4.6. Other risk factors
4.6.1. Geography and Ethnicity. Geographical and ethnic differences may influence the prevalence of KOA. The findings of an epidemiological investigation on OA revealed that the prevalence of KOA was highest among those residing in East Asia (KOA cases >100 millions), followed by South Asia (KOA cases >50 millions), Western Europe (KOA cases >40 millions), and High-income North America (KOA cases >30 millions) [2]. According to a study, it was observed that within the age range of 25-44 years, the prevalence of symptomatic KOA was significantly higher among non-Hispanic whites (970 million) compared to Hispanics (340 million), non-Hispanic blacks (260 million), and non-Hispanic individuals of other racial backgrounds (110 million) in the United States [13].
4.6.2. Occupation Exposure. According to a recent systematic analysis, individuals engaged in physically demanding occupations, such as active military personnel, long-distance runners, football players, wrestlers, weightlifters, farmers and construction workers, exhibit a greater prevalence of KOA. For example, the likelihood of developing KOA was shown to be 3.3-6.9 times higher among male athletes engaged in the above high-intensity conflict sports (mentioned above) compared to their counterparts who did not partake in such sports [14].
4.6.3. Nutritional Supplements. The impact of nutritional factors on OA remains uncertain. A recent meta-analysis has demonstrated that the administration of vitamin D supplements can potentially alleviate pain and slightly improve physical function for individuals with KOA [15]. Furthermore, the utilization of Ginger has been shown to assist persons with KOA in reducing systemic inflammation. The findings indicate a substantial drop (p=0.01) in the value of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, commonly used in the evaluation of systemic inflammation.
5. Prevention
There are three distinct phases to OA prevention: primary, secondary, and tertiary. Firstly, the major objective of primary prevention is to mitigate the risk factors associated with OA for susceptible populations [6]. It is important that some risk factors of KOA can be modified, such as traumatic joint injury, obesity, physically demanding occupations. By contrast, certain risk factors (sex, age, genetic predisposition, and ethnicity) cannot be controlled. But they can serve as criteria for screening individuals who are sensitive to KOA, such as youthful people who are prone to sports-related injuries, the obese, and females. Seriously, the interactions between these risk factors makes the primary phase of OA prevention more difficult. Secondly, the secondary prevention aims to identify and delay the onset of symptomatic OA in susceptible population [6]. At this point, patients have relatively evident knee osteoarthritic symptoms, which are managed through a combination of preventative measures and treatment options. However, a study has pointed out that there are very few clinical trials for this stage [5]. Finally, the tertiary prevention aims to prevent disease progression [5]. At this stage, patient education is very important, especially the prevention of post-traumatic OA (PTOA). Patient education can provide KOA patients aged 15 to 35 who have experienced a sports injury with realistic expectations regarding the timeline for resuming their previous level of physical activity, the likelihood of re-injury, and the most significant risk factors associated with developing KOA. In addition, education can help individuals with KOA comprehend the significance of diet and weight management. The subsequent material is predominately an expansion of primary and secondary prevention.
5.1. Weight loss for KOA
There should be no singular approach to weight loss. Regarding diet, the designers must consider the cultural context and lifestyle habits of adolescents. Because it will affect the procurement and consumption of food by the youth group. It is unknown whether obese children are more likely to develop obesity in young adulthood and middle age. While the prevention of obesity may not directly lead to a reduction in the development of KOA, it can contribute to enhanced physical activity levels and the prevention of various other diseases such as cardiovascular disease, diabetes, and cancer. However, patient education is also highly crucial for weight loss. According to a systematic study, patients’ lack of understanding of the possible health advantages of weight loss, low self-esteem about their weight, and anxiety and powerlessness over attaining weight loss all contribute to failure [16].
5.2. Post-traumatic OA for KOA
The prevention of PTOA aims to prevent joint injuries, delaying the progression of KOA for individuals with previous joint injury. Regarding the prevention of joint injuries, it is noteworthy to consider cruciate ligament injuries, meniscal tears, and intra-articular fractures. These injuries merit attention due to their association with an elevated risk of KOA in young individuals. Regarding the delaying the progression of KOA, it would need to consider more aspects of the mechanisms that are closely associated with this phenomenon include alterations in cartilage metabolism that impact the integrity of joint tissues, inflammation, changes in joint load, epigenetic modifications, and hereditary predisposition [6]. Depending on the patient’s preferences and physical condition, aquatic or land-based activities, such as aerobic exercise, resistance and proprioceptive exercises, and mind-body training, may also be recommended [6]. In addition, it is recommended that patients make certain lifestyle changes and reduce certain predisposing variables, with a particular emphasis on improving patients’ KOA health education.
5.3. Occupational or sports load for KOA
People who engage in physical labor have a very high risk of developing KOA [5]. They should avoid engaging in the same physical activity for an extended period of time at work, such as lifting large objects repeatedly. For the sports pursuits and hobbies of young people, it is prudent to control training load and rest with high-risk sports such as soccer, wrestling and weightlifting. In order to prevent the occurrence of KOA, the trainer should modify the appropriate amount of exercise to prevent joint injury [6].
5.4. Prevention of other modified risk factors for KOA
The combination of risk factors (gender, age, genetic predisposition, and ethnicity) with the characteristics of the KOA epidemic is used to define susceptible populations in order to share them in advance about the symptoms, risks, and prevention strategies of KOA.
6. Conclusion
Among people in the 30-45 age group, the likelihood of developing KOA increases with age. Therefore, the young people who have been exposed to risk factors of KOA for years should be aware of the prevention of KOA. Younger women are at a greater risk than younger males of developing KOA. Overweight children and teenagers are more likely to suffer from KOA. It is essential to require additional research for the effects of these KOA risk factors (age, obesity, genetics, joint trauma, geography, ethnicity, occupation and nutritional supplements) and their interrelationships on young individuals. Prevention measures should focus on the education of KOA-susceptible people or patients with KOA to explain the various KOA risk factors, to help these people establish correct understanding and learn scientific prevention methods. This article only analyses the prevalence and number of people with KOA in three age groups worldwide, and does not analyse whether sample size affects these two indicators. The interaction of the risk factors mentioned above in susceptible populations needs further exploration and research.
References
[1]. Allen K D, Thoma L M, Golightly Y M. Epidemiology of osteoarthritis[J]. Osteoarthritis and cartilage, 2022, 30(2): 184-195.
[2]. Long H, Liu Q, Yin H, et al. Prevalence trends of site‐specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019[J]. Arthritis & Rheumatology, 2022, 74(7): 1172-1183.
[3]. Keramat S A, Alam K, Al-Hanawi M K, et al. Trends in the prevalence of adult overweight and obesity in Australia, and its association with geographic remoteness[J]. Scientific reports, 2021, 11(1): 11320.
[4]. Nedunchezhiyan U, Varughese I, Sun A R J, et al. Obesity, inflammation, and immune system in osteoarthritis[J]. Frontiers in immunology, 2022, 13: 907750.
[5]. Driban J B, Harkey M S, Liu S H, et al. Osteoarthritis and aging: Young adults with osteoarthritis[J]. Current Epidemiology Reports, 2020, 7: 9-15.
[6]. Whittaker J L, Roos E M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury[J]. Best Practice & Research Clinical Rheumatology, 2019, 33(1): 158-171.
[7]. Snoeker B, Turkiewicz A, Magnusson K, et al. Risk of knee OA after different types of knee injuries in young adults: a population-based cohort study[J]. British journal of sports medicine, 2020, 54(12): 725-730.
[8]. Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes (Basel). 2020; 11 (8): 854[J].
[9]. Jenkins W L, Williams III D S B, Williams K, et al. Sex differences in total frontal plane knee movement and velocity during a functional single-leg landing[J]. Physical Therapy in Sport, 2017, 24: 1-6.
[10]. Losina E, Weinstein A M, Reichmann W M, et al. Lifetime risk and age at diagnosis of symptomatic knee OA in the US[J]. Arthritis care & research, 2013, 65(5): 703-711.
[11]. Urban H, Little C B. The role of fat and inflammation in the pathogenesis and management of osteoarthritis[J]. Rheumatology, 2018, 57(suppl_4): iv10-iv21.
[12]. Rice S J, Beier F, Young D A, et al. Interplay between genetics and epigenetics in osteoarthritis[J]. Nature Reviews Rheumatology, 2020, 16(5): 268-281.
[13]. Deshpande B R, Katz J N, Solomon D H, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity[J]. Arthritis care & research, 2016, 68(12): 1743-1750.
[14]. Canetti E F D, Schram B, Orr R M, et al. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A systematic review and meta-analysis[J]. Applied Ergonomics, 2020, 86: 103097.
[15]. Mathieu S, Soubrier M, Peirs C, et al. A meta-analysis of the impact of nutritional supplementation on osteoarthritis symptoms[J]. Nutrients, 2022, 14(8): 1607.
[16]. Lim Y Z, Wong J, Hussain S M, et al. Recommendations for weight management in osteoarthritis: A systematic review of clinical practice guidelines[J]. Osteoarthritis and Cartilage Open, 2022, 4(4): 100298.
Cite this article
Li,K. (2023). Epidemiology of knee Osteoarthritis in the young adults. Theoretical and Natural Science,17,201-208.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Allen K D, Thoma L M, Golightly Y M. Epidemiology of osteoarthritis[J]. Osteoarthritis and cartilage, 2022, 30(2): 184-195.
[2]. Long H, Liu Q, Yin H, et al. Prevalence trends of site‐specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019[J]. Arthritis & Rheumatology, 2022, 74(7): 1172-1183.
[3]. Keramat S A, Alam K, Al-Hanawi M K, et al. Trends in the prevalence of adult overweight and obesity in Australia, and its association with geographic remoteness[J]. Scientific reports, 2021, 11(1): 11320.
[4]. Nedunchezhiyan U, Varughese I, Sun A R J, et al. Obesity, inflammation, and immune system in osteoarthritis[J]. Frontiers in immunology, 2022, 13: 907750.
[5]. Driban J B, Harkey M S, Liu S H, et al. Osteoarthritis and aging: Young adults with osteoarthritis[J]. Current Epidemiology Reports, 2020, 7: 9-15.
[6]. Whittaker J L, Roos E M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury[J]. Best Practice & Research Clinical Rheumatology, 2019, 33(1): 158-171.
[7]. Snoeker B, Turkiewicz A, Magnusson K, et al. Risk of knee OA after different types of knee injuries in young adults: a population-based cohort study[J]. British journal of sports medicine, 2020, 54(12): 725-730.
[8]. Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes (Basel). 2020; 11 (8): 854[J].
[9]. Jenkins W L, Williams III D S B, Williams K, et al. Sex differences in total frontal plane knee movement and velocity during a functional single-leg landing[J]. Physical Therapy in Sport, 2017, 24: 1-6.
[10]. Losina E, Weinstein A M, Reichmann W M, et al. Lifetime risk and age at diagnosis of symptomatic knee OA in the US[J]. Arthritis care & research, 2013, 65(5): 703-711.
[11]. Urban H, Little C B. The role of fat and inflammation in the pathogenesis and management of osteoarthritis[J]. Rheumatology, 2018, 57(suppl_4): iv10-iv21.
[12]. Rice S J, Beier F, Young D A, et al. Interplay between genetics and epigenetics in osteoarthritis[J]. Nature Reviews Rheumatology, 2020, 16(5): 268-281.
[13]. Deshpande B R, Katz J N, Solomon D H, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity[J]. Arthritis care & research, 2016, 68(12): 1743-1750.
[14]. Canetti E F D, Schram B, Orr R M, et al. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: A systematic review and meta-analysis[J]. Applied Ergonomics, 2020, 86: 103097.
[15]. Mathieu S, Soubrier M, Peirs C, et al. A meta-analysis of the impact of nutritional supplementation on osteoarthritis symptoms[J]. Nutrients, 2022, 14(8): 1607.
[16]. Lim Y Z, Wong J, Hussain S M, et al. Recommendations for weight management in osteoarthritis: A systematic review of clinical practice guidelines[J]. Osteoarthritis and Cartilage Open, 2022, 4(4): 100298.









