1. Introduction
In recent couple of decades, incidence and mortality of cancers has been rising alarmingly. Every fourth individual has a lifetime risk of developing cancer, which is a shocking state of affairs [1]. Human have fight with cancers for so many years, currently there many kinds of therapies, including Chemotherapy, Hormone Therapy, Radiation Therapy and so on. However, these conventional therapies have a lot of side effects, some of which are patients suffering from intolerably. The harms of conventional therapies are mainly systemic harms, such as bone marrow suppression, impaired liver and kidney function, gastrointestinal reactions, and cardiotoxicity.
It’s exciting that immunotherapy containing immune checkpoint inhibitors, T-cell transfer therapy, cancer treatment vaccine, gain a successful development over the past few decades. Cancer immunotherapies seek to boost host anti-tumor immunity, alter the tumor's suppressive microenvironment, and eventually reduce tumor burden and improve patient mortality rates. Consequently, compared to other immunotherapies, cancer vaccines provide particular, secure, and tolerable therapy [2]. Cancer vaccines work to treat cancer by teaching the immune system how to identify and respond to antigens associated with tumors and kill cancer cells that carry those antigens [3].
mRNA cancer vaccine, known as a type of nucleic acid vaccine, is a class of cancer treatment vaccine. Some research have shown that mRNA vaccines are effective to treat acute myelocytic leukemia (AML), Glioblastoma (GBM), melanoma, no-small cell lung cancer (NSCLC) and other solid tumors. Although the U.S. FDA recently approved two prophylactic vaccines, one for the human papillomavirus, which is mainly responsible for cervical cancers, and another for the hepatitis B virus, which can cause liver cancer [2], and future cancer therapies may benefit from the therapeutic potential of mRNA vaccines, the majority of mRNA cancer vaccines are still in preclinical or different stages of clinical trials. In addition, some problems remain existing in mRNA cancer vaccines development which form limitation to the application of these vaccines, like disadvantaged methods of mRNA modification and synthesis in vitro, requirement of optimization the delivery systems, mRNAs sensing may dampen immune response, making paradoxical effects to anti-cancer immunotherapy and so on. In this review, this article briefly summarizes how do mRNA cancer vaccines work, their application status and prospects and discuss mainly existing problems in the development of mRNA cancer vaccines and the strategies to solve these problems to improve the therapeutic effect. This review hopes to provide reference and guidelines for the treatment of cancer by mRNA vaccines.
2. Mechanism of mRNA vaccines
mRNAs carried by mRNA cancer vaccines can induce effective antigen-specific immune responses (cellular and humoral immune responses) to realize the anti-tumor goal [4, 5]. The mechanism of mRNA vaccines is divided into 3 major steps:
2.1. mRNA in vitro transcription (IVT)
IVT contains mRNAs synthesis and modification in vitro. One of the most widely used way for producing mRNA is the use of RNA polymerase and linear DNA, which is obtained from linearized plasmid DNA or synthetic DNA produced through PCR. And the technique for producing mRNA in vitro has reached a mature stage, so, it is not the emphasis to discuss in this review.
A mature mRNA has 5 basic structures: a five-prime cap (5′ cap), an open reading frame (ORF) region, 2 untranslated regions (UTRs) and a poly (A) tail [6]. To keep integrate structure benefit mRNA’s stability and expression capability. In addition, the effectiveness of an mRNA vaccine can be improved by further optimized modification on its integrate structure. For example, Moderna mRNA replace uridine with pseudouridine, the substitution of base pairs can decline mRNAs’ immunogenicity. Once accomplishing synthesis and modification, subsequent step needs to go through a series of purification, sterilization, and other processes to remove impurities like enzymes, free nucleotides, residual DNA and foreign RNA fragments, dsRNA and so on.
2.2. mRNA delivery in vivo
After gaining the mature mRNA, the next step is to transport mRNAs into host cells’ cytoplasm so that mRNAs have possibility to play their roles (expressing specific antigens). However, there are some limited factors of mRNA, so mRNAs need some specific delivery systems to deliver mRNAs into numerous host and target cells with sufficiently high translation levels [7, 8] (this paper discusses more details about this problem in the following section.).
2.3. mRNA induce cellular and humoral immune responses
Antigen-presenting cells (APCs) could identify mRNAs. Following cellular uptake by APCs, mRNAs are carried to the cytoplasm through endocytosis and go through antigen processing [5]. mRNAs then prompt pattern recognition receptors (PRRs) to become activated. In cytoplasm, some mRNAs join with host cell ribosomes and carry out effective translation. Antigen proteins can be fragmented by the proteasome into antigenic peptides and then presented to CD8+ T cells via the major histocompatibility complex (MHC) I route to trigger a cellular immune reaction [4]. Additionally, Other antigen proteins may also be secreted from the target cell and ingested by DCs and they are then degraded and given to CD4+ T cells and coactivate antigen-specific B cells via MHC-II pathway to induce humoral immune response [4]. B cells can also recognize secreted antigen proteins, these B cells have the ability to present antigens, and after detecting extracellular proteins and presenting them on their MHC class II surface, they can stimulate CD4+ T cells in return [4, 5].
3. mRNA vaccine advantages
Some research divided cancer vaccines into 4 types: tumor cell- or immune cell-based vaccines, peptide-based vaccines, viral vector vaccines, and nucleic acid (DNA or RNA based) vaccines [9]. Compared with other types of vaccine, mRNA vaccines have a variety of specific advantages in many aspects.
Firstly, nucleic acid vaccines can simultaneously deliver serval antigens like tumor associated antigens (TAAs) or tumor mutations and induce both cellular and humoral immune responses [2]. As a result, nucleic acid vaccines may increase the capacity to against vaccine resistance. Secondly, nucleic acid vaccines contain more completed antigens than peptide-based vaccines, which are beneficial to stimulate a border T cell response [10]. Furthermore, nucleic acid vaccines are also safer than the others because nucleic acid vaccines aren’t exposed to impure or alien proteins and virus-related contaminations.
Although RNA based and DNA based vaccines are both belong to nucleic vaccines, recently mRNA vaccines are proved to have many advanced characteristics over DNA vaccines in cancer treatment. One of the most important reason is that mRNA are similar to mRNA virus in some degree, this characteristic is also known as self-adjuvating properties [4]. So, mRNAs can be recognized by APCs and activate a stronger and durable immune response. In addition, mRNA only need one step of translation to transcript to the target antigens, making it has higher rate of protein than DNA vaccines [2]. The other advantage is that mRNA vaccines are much safer than DNA vaccines. The reason for it is that mRNA is lack of cGp island in structure in comparison with DNA, which means that mRNA never integrate into the genome sequence, thus, mRNA don’t cause gene mutations [2].
4. mRNA vaccines current existing problems and solutions
4.1. Current existing limitations of mRNA vaccine
The reason why not so many mRNA vaccines can be put into clinic application is that preparation of mRNA vaccine faces two mainly problems: one is lacking valid delivery system, the other is that exogenous mRNA may cause immune response termination.
One of the essential requirements of mRNA vaccine to work is that mRNA need to enter the host cytoplasm, so that mRNA can be translated to antigens. However, there are some adverse factors inhibiting mRNA to play a role in host cytoplasm. For instance, mRNA is electronegative molecule, and the same is true for cell membrane, which makes mRNA delivery more difficult. In addition, mRNA is too big to pass through the cell membrane by diffusion [6, 11]. Furthermore, mRNA can be degraded easily by extracellular RNase [6, 11]. Therefore, specific, and efficient mRNA delivery systems need to be designed to carry mRNA into cells.
mRNA vaccine can stimulate immune system to induce strong immune response, but excessively immune response can promote cells to secret a mass of interferons, especially type I IFNs. Some research have shown that CD8+ T cell response can be potentially promoted in moderate type I IFNs degree [12, 13]. But these excessive interferons would restrain mRNA translation and lead to RNA degradation, activated CD8 + T cells depletion, which means mRNA would lose its functions before long [6, 14, 15].
4.2. Solutions to mentioned problems
4.2.1. mRNA delivery systems. Delivery system is necessary for mRNA vaccine to protect it from being degraded by RNase and deliver it to APCs specifically. Currently, many mRNA delivery systems and delivery vehicles (viral- and cell-based vehicles, etc.) have been applied, including delivering naked mRNA, DCs delivery system, protamine delivery system, lipid nanoparticles (LNPs), polymer-based delivery system, etc4. Pual et al. suggested that subcutaneously injecting naked mRNA is more efficient than delivering mRNA through nanoparticle delivery system [16]. Although some studies showed that naked mRNA can be delivered to induce immune response directly, the working efficacy is low, and mRNA is easily degraded after injection.
DCs delivery system is cell-based delivery system, it makes DCs to combine with mRNA transfected production (proteins, peptides, etc.) ex vivo, and then transfer the DC-peptides/proteins combinations into host body to induce immune responses4. This pathway is usually used for cancer treatment because it can induce predominant cellular immune response. However, DCs system is mainly used in pre-clinical experiments because the mRNA transfection rate is quite low [17].
Protamine and polymer (the most widely used material is polyethyleneimine (PEI)), are widely used in clinical trials as mRNA delivery vehicles. Many studies showed that protamine system has potential to deliver mRNA and activate immune response as co-activator, but there are still existing some limitations according to the experimental results [18]. In addition, PEI has been confirmed to have cytotoxicity, which is hard to be metabolized. So, all these delivery pathways still need to be improved due to their limitations.
Compared to aforementioned delivery system, LNPs is more advanced. LNP is made up of ionizable cationic lipids, polyethylene glycol (PEG), phospholipids and cholesterol, and mRNA is wrapped in the central part LNP. LNPs can not only protect mRNA from degradation, but also help mRNA to pass through cell membrane easily by endocytosis (ionizable cationic lipids have contrast chargeability to cell membrane). Geall et al. discovered that mRNA vaccine with LNP can cause higher level of expressed mRNA than injecting naked mRNA and effectively stimulate cellular immune response [19].
4.2.2. mRNA structure optimization. mRNA structures optimization can improve mRNA vaccine efficacy and application. This review has discussed that IFN would induce innate immune reaction to inhibit mRNA translation, and some mRNA structure optimization can prevent these unwanted immune responses. For instance, modifying the 5 'cap is to prevent mRNA from being detected by PRRs and protects mRNA from exonuclease degradation; improvements of ORF encoding the antigen includes the optimization of mRNA codons, which can improve the level of translation by converting less commonly used codons into common codons encoding the same amino acid [20]. Another modification to improve the level of mRNA translation is the substitution of a modified nucleoside which can prevent the recognition of PRRs, allowing the mRNA to produce sufficient levels of protein antigen to trigger an immune response. Moderna's and Pfizer /BioNTech's mRNA COVID-19 vaccines both used modified nucleosides.
4.2.3. utilization of adjuvants. mRNA vaccines combination with certain adjuvants could promote the efficacy of anti-tumor treatment and improve patients’ survival [2]. Toll-like receptors (TLRs) agonists (including TLR4, TLR7/8) could improve the presentation of MHC I in APCs, which can induce a more effective immune response than the mRNA vaccine without using the adjuvant in a mouse model [21]. Miao et al. showed that LNP mRNA cancer vaccine with intrinsic IFN-stimulator of interferon gene can induce a potent and longer cellular response [22]. There are also some studies showing that mRNA vaccines combined with chemokines or pro-inflammatory factors are booming in both pre-clinical experiments and clinical trials [2]. It is exciting that many new mRNA vaccine adjuvants are discovered continuously. For instance, Xiangrong Song et al. in Dec 2022 discovered that Manganese (Mn) can not only improve antigen presenting ability, but also enhance mRNA expression. As an adjuvant of mRNA vaccine, Mn has a broad application prospect [23]. Combination with adjuvants are beneficial for improving mRNA vaccine efficacy and enhancing immune responses, but adjuvants should be used with cautions because they may bring some side effect through interaction with IFN or innate immune pathway, which still needs more study [2].
5. mRNA vaccines application status at present and prospects of future
mRNA vaccine awards the top of the list of " Top Ten Breakthrough Technologies in the World " of MIT Technology Review in 2021. On February 22,2023, Moderna and MSD announced that their mRNA vaccine mRNA-4157, a new tumor antigen, combined with PD-1 antibody to assist in the treatment of high-risk melanoma, has been certified by the FDA as a breakthrough therapy, which is the first mRNA tumor vaccine in the world. However, mRNA cancer vaccines haven’t been approved for standard treatment. Ugur Sahin, co-founder of BioNTech, said: “The launch of mRNA cancer vaccines may wait until around 2030.”
At present, the core targeted antigens of mRNA vaccines are tumor associated antigens (TAAs) and tumor specific neo-epitopes [24, 25]. TAAs are the antigen molecules present on tumor cells, including embryonic proteins, glycoprotein antigens, squamous cell antigens, etc. TAAs are usually related to tumor cell’s abnormal behaviors and proliferation. tumor specific neo-epitopes are a kind of small peptides, which are exposed to the surface of tumor cells, coming from mutation of tumor somatic. These small peptides can be identified by T cells specifically.
LNPs, protamine, etc. are used for mRNA cancer vaccines’ delivery systems and they have been reviewed elsewhere. As for route of administration, mRNA vaccines are usually given through local injection, like s.c., i.m. and i.d. in clinical trials. As reviewed in section 2, mRNA vaccine can induce cellular and humoral immune responses in theory. But now mainly mRNA vaccines’ treatment strategy is stimulating cellular immunity to counter diverse and uncertain cancer genesis. This may lead mRNA cancer vaccine to a valid treatment of cancers [6, 25]. The following diagram show the mRNA vaccines that are in different stages of ongoing clinical trials (Figure 1).
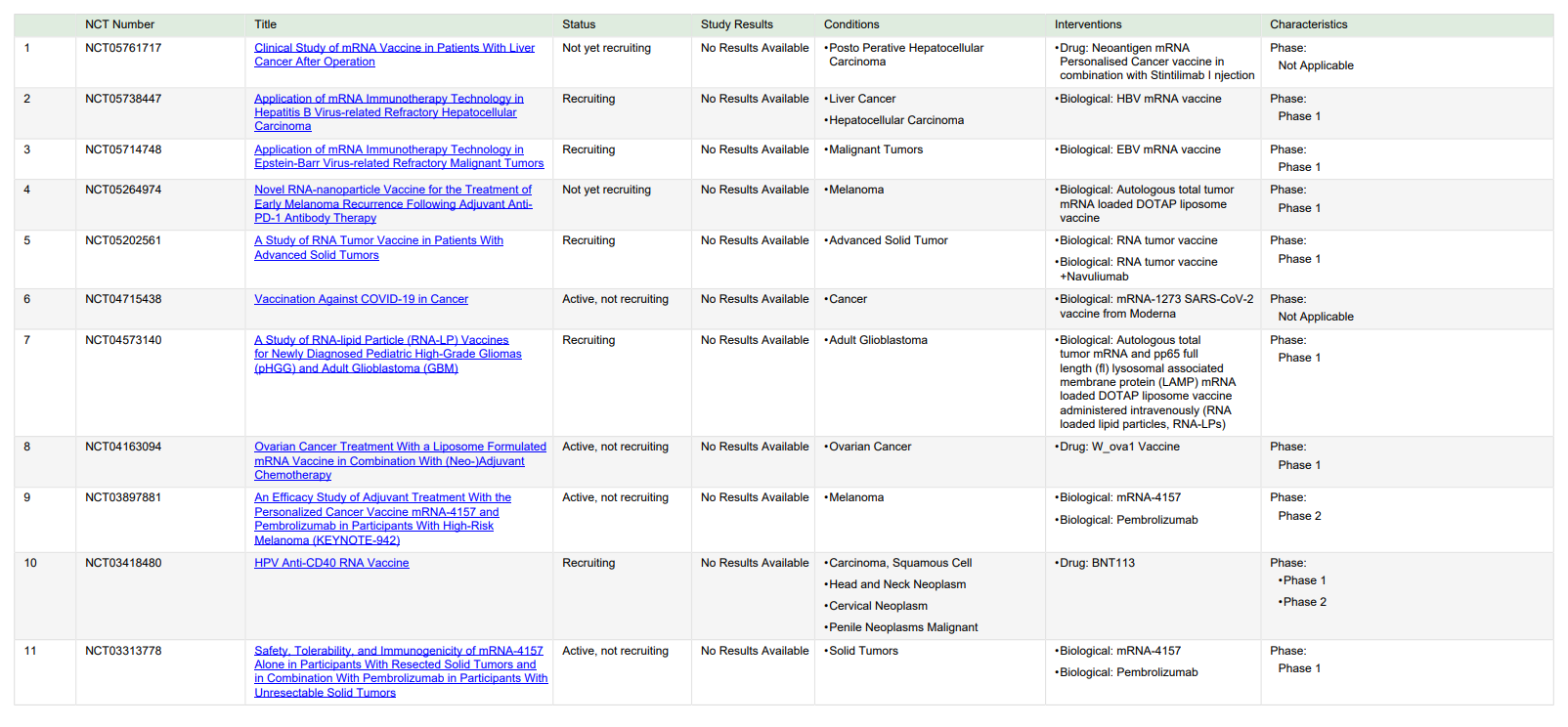
Figure 1. mRNA vaccines in clinical trials. Trials with recruitment status “not yet recruiting,” “recruiting,” and “active, not recruiting” were found on ClinicalTrials.gov with the search terms “cancer” and “RNA, vaccine” on Apr 2, 2023.
Recent studies have had exciting discovery that mRNA vaccines and ICIs in combination can amplify the anti-tumor efficacy. For instance, Ugur Sahin et al. observed in clinical trials that early combination mRNA vaccine with ICIs could more effectively control the progression of breast cancer and melanoma, synergistic play of immunotherapy effect and prevent immune escape [26]. In addition, the maturity of mRNA manufacturing technology allows to produce novel vaccines in a short period of time, which creates possibilities for the use of mRNA vaccines in personalized tumor immunotherapy, although they are still relatively expensive at present. Several clinical studies conducted by BioNTech and Moderna have demonstrated strong anti-tumor immunity using personalized vaccines in several clinical trials for multiple solid tumors, ushering in a new era of therapeutic tumor vaccines. We believe that mRNA tumor therapy vaccines may be a milestone of innovation in cancer immunotherapy. It is expected to be a low-cost, high-efficiency and ideal cancer treatment, which may realize the vision of “a dose for healing cancer”.
6. Conclusion
mRNA vaccines can be synthesized and modified in vitro, and then entered the host body through a specific delivery system to achieve anti-tumor effects by stimulating cellular and humoral immune responses. Compared with other types of vaccine, mRNA vaccine has the advantages of safety, high efficiency, and easy preparation, which make it a promising immunotherapy. However, there are still some limitations in the application of mRNA vaccine at the present stage. For instance, mRNA molecule itself is easy to be degraded by RNase; lacking specific, efficient, and easily degraded delivery systems; moreover, it is easy to stimulate host body to secrete type I IFN, leading to the obstruction of the translation of mRNA molecules, the depletion of T cells, and the termination of immune response. To address these problems, this review summarizes some current solutions, such as modifying the structure of mRNA molecules, adding adjuvants that can improve the therapeutic effect of vaccines, and efficient delivery systems. Although mRNA vaccines are not widely used at the present stage, more and more mRNA vaccines are entering clinical trials. In addition, the recent surprising findings that mRNA vaccines combined with ICIs can greatly enhance the anti-tumor effect. It could be predicted that mRNA vaccine will show its irreplaceable advantages and bright future in the field of cancer immunotherapy, of course, more efforts are needed to solve the existing limitations of mRNA vaccine.
References
[1]. Roy, P. S. and Saikia, B. J., “Cancer and cure: A critical analysis,” Indian J. Cancer 53(3), 441 (2016).
[2]. Miao, L., Zhang, Y. and Huang, L., “mRNA vaccine for cancer immunotherapy,” Mol. Cancer 20(1), 41 (2021).
[3]. “Cancer Treatment Vaccines - Immunotherapy - NCI.”, 24 September 2019, <https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/cancer-treatment-vaccines> (18 March 2023).
[4]. Xu, S., Yang, K., Li, R. and Zhang, L., “mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection,” Int. J. Mol. Sci. 21(18), 6582 (2020).
[5]. Lorentzen, C. L., Haanen, J. B., Met, Ö. and Svane, I. M., “Clinical advances and ongoing trials of mRNA vaccines for cancer treatment,” Lancet Oncol. 23(10), e450–e458 (2022).
[6]. Pardi, N., Hogan, M. J., Porter, F. W. and Weissman, D., “mRNA vaccines - a new era in vaccinology,” Nat. Rev. Drug Discov. 17(4), 261–279 (2018).
[7]. Wadhwa, A., Aljabbari, A., Lokras, A., Foged, C. and Thakur, A., “Opportunities and Challenges in the Delivery of mRNA-Based Vaccines,” 2, Pharmaceutics 12(2), 102 (2020).
[8]. Weng, Y., Li, C., Yang, T., Hu, B., Zhang, M., Guo, S., Xiao, H., Liang, X.-J. and Huang, Y., “The challenge and prospect of mRNA therapeutics landscape,” Biotechnol. Adv. 40, 107534 (2020).
[9]. Faghfuri, E., Pourfarzi, F., Faghfouri, A. H., Abdoli Shadbad, M., Hajiasgharzadeh, K. and Baradaran, B., “Recent developments of RNA-based vaccines in cancer immunotherapy,” Expert Opin. Biol. Ther. 21(2), 201–218 (2021).
[10]. Van Nuffel, A. M. T., Wilgenhof, S., Thielemans, K. and Bonehill, A., “Overcoming HLA restriction in clinical trials,” Oncoimmunology 1(8), 1392–1394 (2012).
[11]. Sahin, U., Karikó, K. and Türeci, Ö., “mRNA-based therapeutics — developing a new class of drugs,” Nat. Rev. Drug Discov. 13(10), 759–780 (2014).
[12]. Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, M., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., et al., “Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy,” Nature 534(7607), 396–401 (2016).
[13]. Broos, K., Van der Jeught, K., Puttemans, J., Goyvaerts, C., Heirman, C., Dewitte, H., Verbeke, R., Lentacker, I., Thielemans, K. and Breckpot, K., “Particle-mediated Intravenous Delivery of Antigen mRNA Results in Strong Antigen-specific T-cell Responses Despite the Induction of Type I Interferon,” Mol. Ther. - Nucleic Acids 5, e326 (2016).
[14]. Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S. and Weissman, D., “Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability,” Mol. Ther. J. Am. Soc. Gene Ther. 16(11), 1833–1840 (2008).
[15]. De Beuckelaer, A., Pollard, C., Van Lint, S., Roose, K., Van Hoecke, L., Naessens, T., Udhayakumar, V. K., Smet, M., Sanders, N., Lienenklaus, S., Saelens, X., Weiss, S., Vanham, G., Grooten, J. and De Koker, S., “Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses,” Mol. Ther. 24(11), 2012–2020 (2016).
[16]. Phua, K. K. L., Leong, K. W. and Nair, S. K., “Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format,” J. Controlled Release 166(3), 227–233 (2013).
[17]. Gay, C. L., DeBenedette, M. A., Tcherepanova, I. Y., Gamble, A., Lewis, W. E., Cope, A. B., Kuruc, J. D., McGee, K. S., Kearney, M. F., Coffin, J. M., Archin, N. M., Hicks, C. B., Eron, J. J., Nicolette, C. A. and Margolis, D. M., “Immunogenicity of AGS-004 Dendritic Cell Therapy in Patients Treated During Acute HIV Infection,” AIDS Res. Hum. Retroviruses 34(1), 111–122 (2018).
[18]. Fotin-Mleczek, M., Duchardt, K. M., Lorenz, C., Pfeiffer, R., Ojkić-Zrna, S., Probst, J. and Kallen, K.-J., “Messenger RNA-based Vaccines With Dual Activity Induce Balanced TLR-7 Dependent Adaptive Immune Responses and Provide Antitumor Activity,” Journal of Immunotherapy 34(1), 1–15 (2011).
[19]. Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., Cu, Y., Beard, C. W., Brito, L. A., Krucker, T., O’Hagan, D. T., Singh, M., Mason, P. W., Valiante, N. M., Dormitzer, P. R., Barnett, S. W., Rappuoli, R., Ulmer, J. B. and Mandl, C. W., “Nonviral delivery of self-amplifying RNA vaccines,” Proc. Natl. Acad. Sci. 109(36), 14604–14609 (2012).
[20]. Linares-Fernández, S., Lacroix, C., Exposito, J.-Y. and Verrier, B., “Tailoring mRNA Vaccine to Balance nnate/Adaptive Immune Response,” Trends Mol. Med. 26(3), 311–323 (2020).
[21]. Islam, M. A., Rice, J., Reesor, E., Zope, H., Tao, W., Lim, M., Ding, J., Chen, Y., Aduluso, D., Zetter, B. R., Farokhzad, O. C. and Shi, J., “Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and herapeutic tumor suppression in mice,” Biomaterials 266, 120431 (2021).
[22]. Miao, L., Lin, J., Huang, Y., Li, L., Delcassian, D., Ge, Y., Shi, Y. and Anderson, D. G., “Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver,” Nat. Commun. 11(1), 2424 (2020).
[23]. Fan, N., Chen, K., Zhu, R., Zhang, Z., Huang, H., Qin, S., Zheng, Q., He, Z., He, X., Xiao, W., Zhang, Y., Gu, Y., Zhao, C., Liu, Y., Jiang, X., Li, S., Wei, Y. and Song, X., “Manganese-coordinated mRNA vaccines with enhanced mRNA expression and immunogenicity induce robust immune responses against SARS-CoV-2 variants,” Sci. Adv. 8(51), eabq3500.
[24]. Kreiter, S., Castle, J. C., Türeci, Ö. and Sahin, U., “Targeting the tumor mutanome for personalized vaccination herapy,” OncoImmunology 1(5), 768–769 (2012).
[25]. Sahin, U., Derhovanessian, E., Miller, M., Kloke, B.-P., Simon, P., Löwer, M., Bukur, V., Tadmor, A. D., Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr, M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck, J., Heesch, S., Schreeb, K. H., Müller, F., et al., “Personalized RNA mutanome vaccines mobilize poly-specific herapeutic immunity against cancer,” Nature 547(7662), 222–226 (2017).
[26]. Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., Maurus, D., Schwarck-Kokarakis, D., Kuhn, A. N., Omokoko, T., Kranz, L. M., Diken, M., Kreiter, S., Haas, H., Attig, S., Rae, R., Cuk, K., Kemmer-Brück, A., Breitkreuz, A., et al., “An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma,” 7823, Nature 585(7823), 107–112 (2020).
Cite this article
Zeng,X. (2023). Progress in mRNA cancer vaccine research, current limitations, application and future. Theoretical and Natural Science,20,141-147.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Roy, P. S. and Saikia, B. J., “Cancer and cure: A critical analysis,” Indian J. Cancer 53(3), 441 (2016).
[2]. Miao, L., Zhang, Y. and Huang, L., “mRNA vaccine for cancer immunotherapy,” Mol. Cancer 20(1), 41 (2021).
[3]. “Cancer Treatment Vaccines - Immunotherapy - NCI.”, 24 September 2019, <https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/cancer-treatment-vaccines> (18 March 2023).
[4]. Xu, S., Yang, K., Li, R. and Zhang, L., “mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection,” Int. J. Mol. Sci. 21(18), 6582 (2020).
[5]. Lorentzen, C. L., Haanen, J. B., Met, Ö. and Svane, I. M., “Clinical advances and ongoing trials of mRNA vaccines for cancer treatment,” Lancet Oncol. 23(10), e450–e458 (2022).
[6]. Pardi, N., Hogan, M. J., Porter, F. W. and Weissman, D., “mRNA vaccines - a new era in vaccinology,” Nat. Rev. Drug Discov. 17(4), 261–279 (2018).
[7]. Wadhwa, A., Aljabbari, A., Lokras, A., Foged, C. and Thakur, A., “Opportunities and Challenges in the Delivery of mRNA-Based Vaccines,” 2, Pharmaceutics 12(2), 102 (2020).
[8]. Weng, Y., Li, C., Yang, T., Hu, B., Zhang, M., Guo, S., Xiao, H., Liang, X.-J. and Huang, Y., “The challenge and prospect of mRNA therapeutics landscape,” Biotechnol. Adv. 40, 107534 (2020).
[9]. Faghfuri, E., Pourfarzi, F., Faghfouri, A. H., Abdoli Shadbad, M., Hajiasgharzadeh, K. and Baradaran, B., “Recent developments of RNA-based vaccines in cancer immunotherapy,” Expert Opin. Biol. Ther. 21(2), 201–218 (2021).
[10]. Van Nuffel, A. M. T., Wilgenhof, S., Thielemans, K. and Bonehill, A., “Overcoming HLA restriction in clinical trials,” Oncoimmunology 1(8), 1392–1394 (2012).
[11]. Sahin, U., Karikó, K. and Türeci, Ö., “mRNA-based therapeutics — developing a new class of drugs,” Nat. Rev. Drug Discov. 13(10), 759–780 (2014).
[12]. Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., Meng, M., Fritz, D., Vascotto, F., Hefesha, H., Grunwitz, C., Vormehr, M., Hüsemann, Y., Selmi, A., Kuhn, A. N., Buck, J., Derhovanessian, E., Rae, R., Attig, S., et al., “Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy,” Nature 534(7607), 396–401 (2016).
[13]. Broos, K., Van der Jeught, K., Puttemans, J., Goyvaerts, C., Heirman, C., Dewitte, H., Verbeke, R., Lentacker, I., Thielemans, K. and Breckpot, K., “Particle-mediated Intravenous Delivery of Antigen mRNA Results in Strong Antigen-specific T-cell Responses Despite the Induction of Type I Interferon,” Mol. Ther. - Nucleic Acids 5, e326 (2016).
[14]. Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S. and Weissman, D., “Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability,” Mol. Ther. J. Am. Soc. Gene Ther. 16(11), 1833–1840 (2008).
[15]. De Beuckelaer, A., Pollard, C., Van Lint, S., Roose, K., Van Hoecke, L., Naessens, T., Udhayakumar, V. K., Smet, M., Sanders, N., Lienenklaus, S., Saelens, X., Weiss, S., Vanham, G., Grooten, J. and De Koker, S., “Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses,” Mol. Ther. 24(11), 2012–2020 (2016).
[16]. Phua, K. K. L., Leong, K. W. and Nair, S. K., “Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format,” J. Controlled Release 166(3), 227–233 (2013).
[17]. Gay, C. L., DeBenedette, M. A., Tcherepanova, I. Y., Gamble, A., Lewis, W. E., Cope, A. B., Kuruc, J. D., McGee, K. S., Kearney, M. F., Coffin, J. M., Archin, N. M., Hicks, C. B., Eron, J. J., Nicolette, C. A. and Margolis, D. M., “Immunogenicity of AGS-004 Dendritic Cell Therapy in Patients Treated During Acute HIV Infection,” AIDS Res. Hum. Retroviruses 34(1), 111–122 (2018).
[18]. Fotin-Mleczek, M., Duchardt, K. M., Lorenz, C., Pfeiffer, R., Ojkić-Zrna, S., Probst, J. and Kallen, K.-J., “Messenger RNA-based Vaccines With Dual Activity Induce Balanced TLR-7 Dependent Adaptive Immune Responses and Provide Antitumor Activity,” Journal of Immunotherapy 34(1), 1–15 (2011).
[19]. Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., Cu, Y., Beard, C. W., Brito, L. A., Krucker, T., O’Hagan, D. T., Singh, M., Mason, P. W., Valiante, N. M., Dormitzer, P. R., Barnett, S. W., Rappuoli, R., Ulmer, J. B. and Mandl, C. W., “Nonviral delivery of self-amplifying RNA vaccines,” Proc. Natl. Acad. Sci. 109(36), 14604–14609 (2012).
[20]. Linares-Fernández, S., Lacroix, C., Exposito, J.-Y. and Verrier, B., “Tailoring mRNA Vaccine to Balance nnate/Adaptive Immune Response,” Trends Mol. Med. 26(3), 311–323 (2020).
[21]. Islam, M. A., Rice, J., Reesor, E., Zope, H., Tao, W., Lim, M., Ding, J., Chen, Y., Aduluso, D., Zetter, B. R., Farokhzad, O. C. and Shi, J., “Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and herapeutic tumor suppression in mice,” Biomaterials 266, 120431 (2021).
[22]. Miao, L., Lin, J., Huang, Y., Li, L., Delcassian, D., Ge, Y., Shi, Y. and Anderson, D. G., “Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver,” Nat. Commun. 11(1), 2424 (2020).
[23]. Fan, N., Chen, K., Zhu, R., Zhang, Z., Huang, H., Qin, S., Zheng, Q., He, Z., He, X., Xiao, W., Zhang, Y., Gu, Y., Zhao, C., Liu, Y., Jiang, X., Li, S., Wei, Y. and Song, X., “Manganese-coordinated mRNA vaccines with enhanced mRNA expression and immunogenicity induce robust immune responses against SARS-CoV-2 variants,” Sci. Adv. 8(51), eabq3500.
[24]. Kreiter, S., Castle, J. C., Türeci, Ö. and Sahin, U., “Targeting the tumor mutanome for personalized vaccination herapy,” OncoImmunology 1(5), 768–769 (2012).
[25]. Sahin, U., Derhovanessian, E., Miller, M., Kloke, B.-P., Simon, P., Löwer, M., Bukur, V., Tadmor, A. D., Luxemburger, U., Schrörs, B., Omokoko, T., Vormehr, M., Albrecht, C., Paruzynski, A., Kuhn, A. N., Buck, J., Heesch, S., Schreeb, K. H., Müller, F., et al., “Personalized RNA mutanome vaccines mobilize poly-specific herapeutic immunity against cancer,” Nature 547(7662), 222–226 (2017).
[26]. Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., Maurus, D., Schwarck-Kokarakis, D., Kuhn, A. N., Omokoko, T., Kranz, L. M., Diken, M., Kreiter, S., Haas, H., Attig, S., Rae, R., Cuk, K., Kemmer-Brück, A., Breitkreuz, A., et al., “An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma,” 7823, Nature 585(7823), 107–112 (2020).









