1. Introduction
1.1. History of Epinephrine
Epinephrine is a hormone secreted by the adrenal glands with a broad application in the medical industry. From the last half of the 19th century to the early start of the 20th century, scientists from different regions of the world conducted investigations relating to adrenal glands and their secretion product, epinephrine. After the first observation of adrenal glands in 1855 by Thomas Addison, noticing the significance of such an organ led to further investigation. It was not until the late 19th century where English physiologists George Oliver and Sir Edward Albert Sharpey-Schafer discovered the effects of epinephrine and described it as the “blood-pressure-raising effect of a substance from the adrenal medulla”. Entering the new century, epinephrine was isolated and identified by Jokichi Takamine, a Japanese American biochemist, and the synthesis of a ketone form of epinephrine was later developed by Friedrich Stolz and Henry Drysdale Dakin in 1904 [1-4]. The final conversion of adrenaline into epinephrine was finally created in 1906, becoming the first laboratory-synthesized hormone [5].
1.2. General Information on Epinephrine
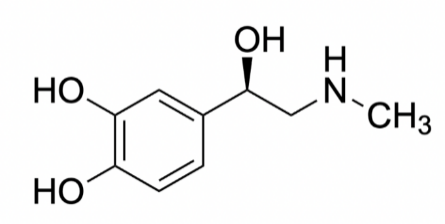
Figure 1. Structure of epinephrine
Epinephrine, also referred to as adrenaline, acts as both a hormone and a neurotransmitter. On top of each kidney, our adrenal glands produce and release this hormone. Epinephrine is also a neurotransmitter in the central nervous system and facilitates the transmission of nerve signals from one nerve cell, muscle cell, or gland cell to another. This chemical is associated with the sympathetic nervous system, responsible for the “fight-or-flight” response to danger. Irregular epinephrine levels have been linked to hypertension, anxiety, sleep disorders, and reduced immunity [6]. Adrenaline affects the body in six significant ways. First, it widens the air passages to supply muscles with the oxygen necessary to fight or flee danger. Second, it constricts blood vessels and redirects blood flow to critical muscle groups like the heart and lungs. Third, it accelerates heart rate and increases heart contraction force, delivering oxygen to muscles and tissues. Fourth, it prompts the liver to release glucose into the bloodstream, providing energy to the body. Fifth, it causes pupils to dilate, improving vision even in low-light conditions. Lastly, it reduces pain perception, enabling people to continue fighting or fleeing, even when injured. Adrenaline is released in response to real threats and during moments of emotional stress, such as taking tests, public speaking, dating, watching a scary movie, or participating in extreme sports like skydiving [7]. Synthetic epinephrine, when used medicinally, is employed in treating various conditions, such as cardiac arrest and cardiopulmonary resuscitation (CPR), eye surgery, septic shock, asthma, and anaphylaxis. Epinephrine is used to stimulate the heart during CPR, help maintain pupil dilation during eye surgery, and raise blood pressure in cases of septic shock. Additionally, it helps open airways, reduces airway spasms in those with asthma, and relaxes airway muscles in individuals experiencing anaphylaxis, a severe and potentially deadly allergic reaction [8].
Epinephrine is a white to brownish crystalline powder, which is odorless and has a bitter taste. Adrenaline is soluble in water, ethanol, and chloroform, but it is insoluble in ether and benzene [9]. It has a melting point of around 215-217 degrees Celsius, and its boiling point is approximately 300 degrees Celsius [10]. Adrenaline is highly reactive, and its chemical properties allow it to bind to specific receptors on the surface of cells, including the heart, lungs, and blood vessels. Figure 1 shows the molecular structure of epinephrine that allows it to acquire its corresponding chemical properties.
1.2.1. General Chemical Information about Epinephrine. The molecular formula of adrenaline, also known as epinephrine, is C9H13NO3. It is a molecule with a nominal mass of 183 and a total mass of 183.089543 [10]. Adrenaline comprises carbon, hydrogen, nitrogen, and oxygen atoms and contains 26 covalent bonds forming a covalent molecule. It includes 13 non-hydrogen bonds and 6 hybrid C-C bonds that form the 6-membered ring. 6 of the carbon atoms in the molecule are sp2 bonded, while the other 3 of the carbon atoms are sp3 bonded. The oxygen and nitrogen are all sp3 bonding as oxygen has 2 bond pair and 2 lone pair, and nitrogen has 3 bond pair and 1 lone pair [11].
1.2.2. Structure of Epinephrine. The backbone structure of adrenaline is formed by covalent bonds between carbon and hydrogen atoms, and it also has polar covalent bonds between oxygen and hydrogen atoms that make it soluble in water. Adrenaline has an amine group (-NH2) and a catechol group (-C6H4OH), connected by a single carbon atom forming a peptide bond. (See figure 1.) The functional groups present in adrenaline, like the amine, catechol, and carboxylic acid groups, enable it to interact with specific receptors on the surface of cells in the body [12].
2. Synthesis of Epinephrine
2.1. Biosynthesis of Epinephrine
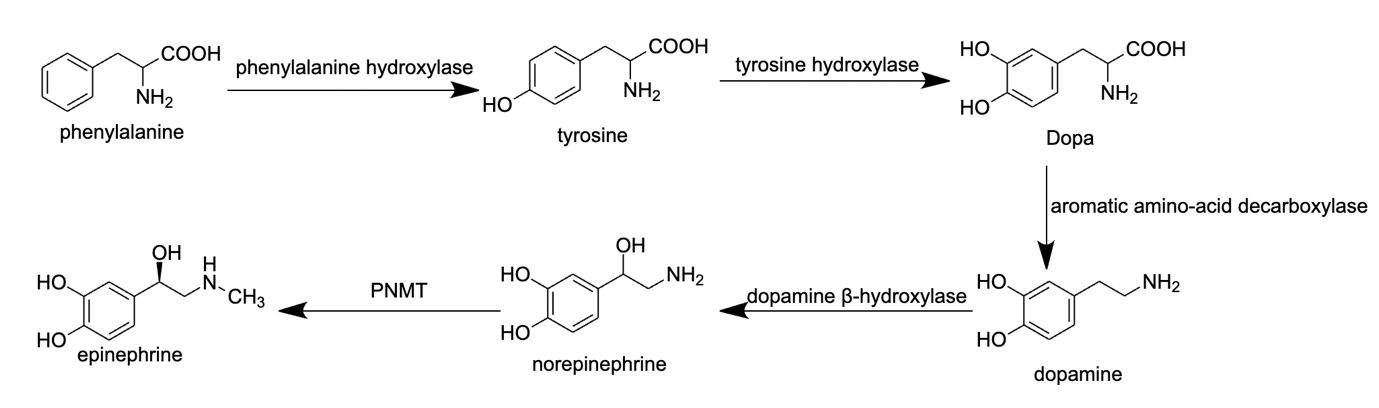
Figure 2. Process of epinephrine biosynthesis
2.1.1. The Process of Phenylalanine to Tyrosine. There are 5 steps in epinephrine biosynthesis, as shown in Figure 2. Phenylalanine is an amino acid that can be converted to tyrosine in phenylalanine hydroxylation, the first step of biosynthesis. This process involves the enzyme phenylalanine hydroxylase, which converts a hydroxyl group to the phenylalanine molecule into tyrosine [13].
2.1.2. The Process of Tyrosine to Dopa. The conversion of tyrosine to DOPA (3,4-dihydroxyphenylalanine) is the second step to form epinephrine. The reaction is catalysed by the enzyme tyrosine hydroxylase, which adds a hydroxyl group (-OH) to the carbon atom adjacent to the amino group (-NH2) of tyrosine [14-15].
2.1.3. The Process of Dopa to Dopamine. DOPA can be further metabolized to dopamine by the enzyme aromatic L-amino acid decarboxylase, which removes the carboxyl group (-COOH) from DOPA [14-15].
2.1.4. The Process of Dopamine to Norepinephrine. The conversion of dopamine to norepinephrine is the 4th step of epinephrine biosynthesis. The reaction is catalysed by the enzyme dopamine beta-hydroxylase, which adds a hydroxyl group (-OH) to the carbon atom adjacent to the amino group (-NH2) of dopamine, forming norepinephrine [16].
2.1.5. The Process of Norepinephrine to Epinephrine. The last step in this biosynthesis is norepinephrine to epinephrine. The reaction is catalysed by the enzyme phenylethanolamine N-methyltransferase (PNMT), which transfers a methyl group (-CH3) from S-adenosyl-L-methionine (SAM) to the nitrogen atom (-NH2) of norepinephrine, forming epinephrine [15].
2.2. Chemical Synthesis of Epinephrine
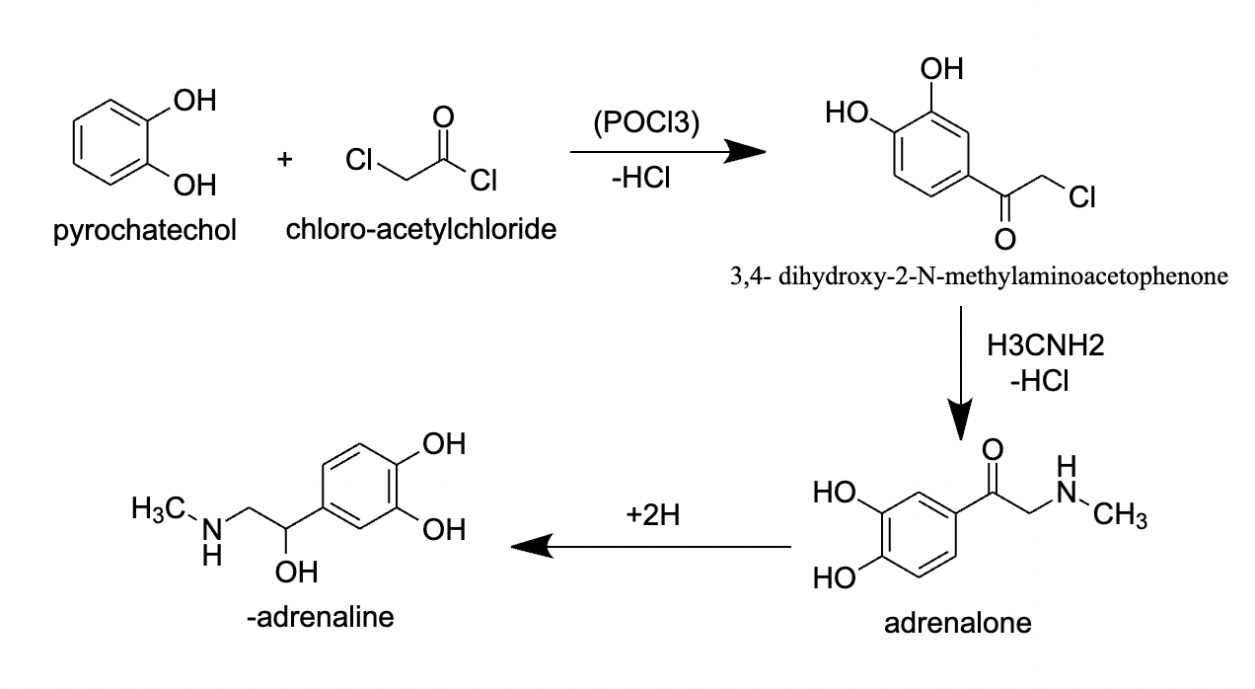
Figure 3. Process of chemical synthesis of epinephrine
Another way to synthesize epinephrine chemically is the Enantioselective Synthesis method, as shown in Figure 3. In this method, reacting pyrocatechol with chloro-acetylchloride under strong hydrochloric acid would form 3’,4’- dihydroxy-2-N-methylaminoacetophenone. Then, this intermediate is used to produce epinephrine by hydrogenation by using about a 50-bar hydrogen pressure, with a chiral hydroxyalkyl ferrocenyl phosphine as a catalyst. However, this process usually takes about 2-4 days, which makes it not suitable for the industrial synthesis of epinephrine, so other modern syntheses of epinephrine, like asymmetric hydrogenation, are developed to become a more efficient method of the synthesis of epinephrine [17].
3. Function and Metabolism of Epinephrine in the body
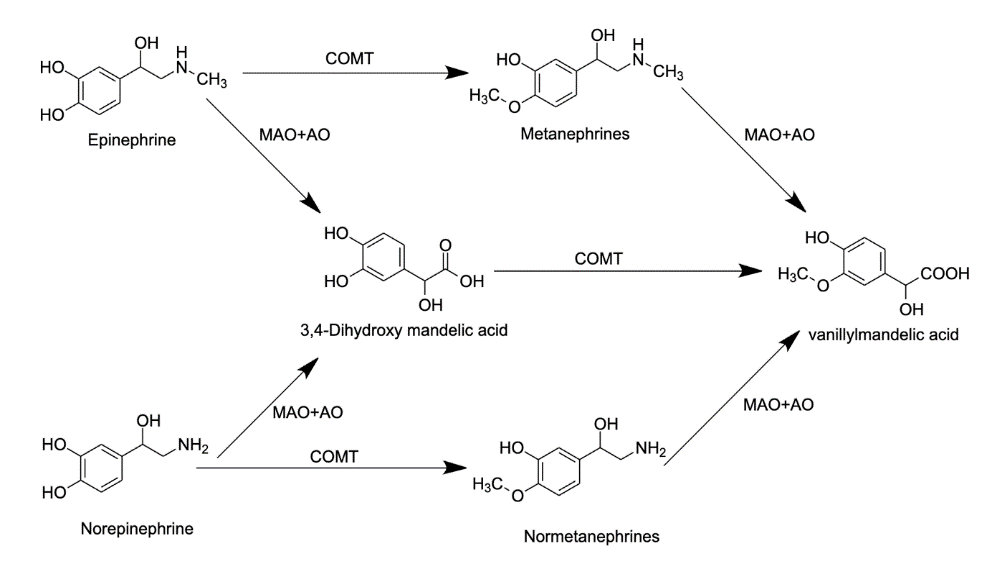
Figure 4. Process of metabolism of epinephrine
The synthesis process of epinephrine starts with the amino acid tyrosine, going through the whole synthesis process and at last, forming epinephrine, norepinephrine, and dopamine. These three hormones are all stored inside the adrenal glands with different usages for the body. The normal epinephrine level inside an average adult is 0 to 140 g/Ml. For norepinephrine, the intermediate level is 70 to 1700 pg/mL inside an average adult, while the expected level of dopamine inside an average adult is only 0 to 30 pg/mL [18]. With too many of these hormones in the body, the different body functions would get boosted over the limit, adding risks towards various cardiovascular diseases like heart attack, heart palpitations, and high blood pressure. So the human body stores epinephrine parts and produces more under multiple situations. The rate of epinephrine synthesized in the body is also fast, usually taking about 2-3 minutes from the production to feel the effect of epinephrine [19]. After epinephrine is used in the body, it takes about 1 hour for the effects to wear off. At the same time, during that period, the user might have a headache, hypertension, weakness, tremors, and vomit. While the effects of epinephrine wear off, the hormone also gets converted into a vanillic acid. As shown in Figure 4, the conversion can happen in two ways, first epinephrine may metabolize with monoamine oxidase and aldehyde oxidase enzymes, forming 3,4-Dihydoxy mandelic acid, which then metabolizes with catechol-O-methyltransferase enzyme forming into vanillylmandelic acid which then gets excreted out of the body through urine. Another way that metabolizes epinephrine into vanillylmandelic acid is using catechol-O-methyltransferase enzyme, which metabolizes epinephrine into metanephrine, then using monoamine oxidase and aldehyde oxidase enzymes to metabolize into vanillylmandelic acid. The metabolism of norepinephrine into vanillylmandelic acid has a similar procedure to the metabolism of epinephrine [20].
4. Difference and Similarity Between Epinephrine and Norepinephrine
Epinephrine and norepinephrine are catecholamines, a compound that functions as neurotransmitters and hormones. Comparing these two molecules reveals both similarities and differences. Both epinephrine and norepinephrine are catecholamines that affect the heart, blood sugar levels, and blood vessels. While they have similar effects, norepinephrine can cause vasoconstriction, narrowing blood vessels and increasing blood pressure. Epinephrine, or adrenaline, is a powerful hormone with significant physiological effects. These effects include increased blood glucose levels, elevated heart rate, more muscular heart contractions, and relaxation of airway muscles to facilitate breathing. These actions provide the body with extra energy. In times of severe stress or fear, the body releases a surge of epinephrine, triggering the fight-or-flight response. Norepinephrine has similar effects to epinephrine, such as increased blood glucose levels, elevated heart rate, and more muscular contractions. However, norepinephrine also causes vasoconstriction, narrowing blood vessels and increasing blood pressure. Additionally, they differ in their applications. Epinephrine is a synthetic form of the hormone and neurotransmitter used in medical treatments. It is primarily used for anaphylaxis, severe asthma attacks, cardiac arrest, severe infections, and as an additive to local anesthetics. It acts quickly to improve breathing, stimulate the heart, raise blood pressure, and alleviate allergic reactions. Norepinephrine treats septic shock, a severe bacterial infection that can cause organ failure and low blood pressure. It is administered intravenously to constrict blood vessels and increase blood pressure. Norepinephrine is preferred over epinephrine due to its specific alpha-receptor activity. Some individuals with depression or attention deficit hyperactivity disorder (ADHD) take medications that stimulate or increase the release of norepinephrine, such as atomoxetine and serotonin-norepinephrine reuptake inhibitors (SNRIs) like duloxetine (Cymbalta) and venlafaxine (Effexor XR) [21].
5. Conclusion
Epinephrine has been used for different medical purposes since its discovery. The first use of epinephrine in medical surgery was in 1896, when an American physician William H. Bates used epinephrine for eye surgeries [22-23]. It was later used to treat a severe allergic known as Anaphylaxis. For this allergic sickness, a modern technology treatment called EpiPen was developed in the mid-1970s by Sheldon Kaplan in survival technology as the first modern epinephrine auto-injector. It was later approved to sell in the market in 1987, and since then, it has saved numerous lives that suffer from Anaphylaxis. Some other medical use of epinephrine includes cardiac arrest, croup, asthma and other life-threatening physical damage on the body. This is due to epinephrine’s effect on the body, as it is able to reduce the amount of blood loss, improve breathing, stimulate the heart, raise dropping blood pressure, reverse hives and reduce swelling [24-25]. While epinephrine has a broad use in the medical industry now on physical illnesses, it may also have a variety of future applications on mental health and development. MIT researchers have used rodents to stimulate the adrenal glands and control the amount of hormones released that link with stress. [26] These hormones include dopamine, norepinephrine and epinephrine which are intermediates or products of the synthesis of epinephrine. This shows the possibility of using epinephrine as medicine for curing mental sicknesses like anxiety and depression. The release of epinephrine also increases level of functions in the limbic system, including the hypothalamus, the hippocampus, and the amygdala. These parts of the brain control emotional responses, behaviour, and long-term memory, which shows that the release of epinephrine is associated with improving memories and other brain functions. This also shows the potential of epinephrine to improve brain functions significantly or even develop new brain functions that may evolve human beings [27-28].
Acknowledgement
Wenqi Deng, Yujing Feng and Chi Yuan Henry Zhai contributed equally to this work and should be considered co-first authors.
References
[1]. Grossman, A. (2009, July 25). The Discovery Of Adrenaline. BrainImmune - Trends in Neuroendocrine Immunology. https://brainimmune.com/the-discovery-of-adrenaline/#:~:text=Friedrich%20Stolz%20at%20Farbwerke%20Hochst,in%20the%20laboratory%20%5B6%5D.
[2]. Hoffman, B. (2016). Adrenaline. Serious Science. https://serious-science.org/adrenaline-7601#:~:text=A%20number%20of%20people%20claimed,He%20named%20it%20’adrenaline’.
[3]. Epinephrine | Description, Production, & Function | Britannica. (2023). In Encyclopædia Britannica. https://www.britannica.com/science/epinephrine
[4]. all, C. M., & Featherstone, P. J. (2017). The Early History of Adrenaline. Anaesthesia and Intensive Care, 45(3), 279–281. https://doi.org/10.1177/0310057x1704500301
[5]. Sneader, W. (2001). The discovery and synthesis of epinephrine. Drug News & Perspectives, 14(8), 491. https://doi.org/10.1358/dnp.2001.14.8.858417
[6]. Epinephrine (Adrenaline): What It Is, Function, Deficiency & Side Effects. (2022). Cleveland Clinic. https://my.clevelandclinic.org/health/articles/22611-epinephrine-adrenaline
[7]. https://www.facebook.com/verywell. (2021). What Is Adrenaline? Verywell Health. https://www.verywellhealth.com/what-is-adrenaline-5094550
[8]. Epinephrine inhalation aerosol | Cleveland Clinic. (2023). Cleveland Clinic. https://my.clevelandclinic.org/health/drugs/19722-epinephrine-inhalation-aerosol
[9]. Office, N. (2020). EPINEPHRINE | CAMEO Chemicals | NOAA. Noaa.gov. https://cameochemicals.noaa.gov/chemical/16207
[10]. PubChem. (2023). Hazardous Substances Data Bank (HSDB): 4289. @Pubchem; PubChem. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/4289
[11]. 2D Chemical Structure Image of (S)-adrenaline on Its Chemical Structure Page. (2022). Mol-Instincts. https://www.molinstincts.com/structure/S-adrenaline-cstr-CT1002341597.html #:~:text=The%20(S)%2Dadrenaline%20molecule,1%20secondary%20alcohol(s).
[12]. 2D Chemical Structure Image of Epinephrine on Its Chemical Structure Page. (2022). Mol-Instincts. https://www.molinstincts.com/structure/epinephrine-cstr-CT1002033985.html
[13]. Nutr, J. (n.d.). An Overview of Phenylalanine and Tyrosine Kinetics in Humans. National Library of Medicine. Retrieved March 26, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2268015/
[14]. Pharmacology Animation. (n.d.). YouTube [YouTube]. https://youtu.be/H1qUpuf0ZP4
[15]. Pelto, L., B.D Byylund, Salminen, S., & Isolauri, E. (n.d.). Epinephrine. ScienceDirect. Retrieved March 26, 2023, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/epinephrine
[16]. Sykora, M., Schönenberger, S. and Bösel, J. Sykora, M., Schönenberger, S., & Bösel, J. (2016). (n.d.). Norepinephrine. ScienceDirect. Retrieved March 26, 2023, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/
[17]. Wolter, L., & Dhaun, H. (2001). https://patentimages.storage.googleapis.com/03/d5/1a/04a1f 96cbe6f72/US6218575.pdf
[18]. Catecholamine blood test Information | Mount Sinai - New York. (2013). Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/catecholamine-blood-test#:~:text= Normal%20Results,(764.3%20pmol%2FL).
[19]. Adrenaline. (2023, January 27). Healthdirect.gov.au; Healthdirect Australia. https://www.healthdirect.gov.au/adrenaline#:~:text=This%20is%20known%20as%20the,within%202%20or%203%20minutes.
[20]. Pelto, L., Salminen, S., & Isolauri, E. (2002). MILK ALLERGY. Encyclopedia of Dairy Sciences, 1821–1827. https://doi.org/10.1016/b0-12-227235-8/00306-0
[21]. Nall, R. (2018, February 15). What’s the Difference Between Epinephrine and Norepinephrine? Healthline; Healthline Media. https://www.healthline.com/health/epinephrine-vs-norepinephrine#uses
[22]. William_Bates. (2023). Bionity.com. https://www.bionity.com/en/encyclopedia/ William_Bates. html
[23]. The Use of Extract of Suprarenal Capsule in the Eye - www.Central-Fixation.com. (2023). Central-Fixation.com. https://www.central-fixation.com/bates-medical-articles/use-of-extract -of-suprarenal-capsule.php
[24]. Hassanpour, S. E., Zirakzadeh, H., & Aghajani, Y. (2020). The Effect of Subcutaneous Epinephrine Dosage on Blood Loss in Surgical Incisions. WORLD JOURNAL of PLASTIC SURGERY, 9(3), 309–312. https://doi.org/10.29252/wjps.9.3.309
[25]. Epinephrine Intramuscular: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD. (2023). Webmd.com. https://www.webmd.com/drugs/2/drug-93171/epinephrine- intramuscular/details#:~:text=This%20medication%20is%20used%20in,face%2C%20lips%2C%20and%20throat.
[26]. Trafton, A. (2020, April). Researchers achieve remote control of hormone release. MIT News | Massachusetts Institute of Technology. https://news.mit.edu/2020/remote-control-hormone-release-nanoparticles-0410
[27]. Fitzgerald, M. (2008, August 27). A Mixed Blessing for Memory: Stress and the Brain - Brain Connection. Brain Connection. https://brainconnection.brainhq.com/2008/08/26/a-mixed-blessing-for-memory-stress-and-the-brain/#:~:text=The%20limbic%20system%20includes%20the,been%20associated%20with%20improved%20memory.
[28]. Hypothalamic-Pituitary-Adrenal (HPA) Axis. (n.d.). https://www.unh.edu/ pacs/sites/default/files/media/2020-07/stress-and-your-body-handout.pdf
Cite this article
Deng,W.;Feng,Y.;Zhai,C.Y.H. (2023). Investigation of epinephrine molecule and its synthesis. Theoretical and Natural Science,22,296-302.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Grossman, A. (2009, July 25). The Discovery Of Adrenaline. BrainImmune - Trends in Neuroendocrine Immunology. https://brainimmune.com/the-discovery-of-adrenaline/#:~:text=Friedrich%20Stolz%20at%20Farbwerke%20Hochst,in%20the%20laboratory%20%5B6%5D.
[2]. Hoffman, B. (2016). Adrenaline. Serious Science. https://serious-science.org/adrenaline-7601#:~:text=A%20number%20of%20people%20claimed,He%20named%20it%20’adrenaline’.
[3]. Epinephrine | Description, Production, & Function | Britannica. (2023). In Encyclopædia Britannica. https://www.britannica.com/science/epinephrine
[4]. all, C. M., & Featherstone, P. J. (2017). The Early History of Adrenaline. Anaesthesia and Intensive Care, 45(3), 279–281. https://doi.org/10.1177/0310057x1704500301
[5]. Sneader, W. (2001). The discovery and synthesis of epinephrine. Drug News & Perspectives, 14(8), 491. https://doi.org/10.1358/dnp.2001.14.8.858417
[6]. Epinephrine (Adrenaline): What It Is, Function, Deficiency & Side Effects. (2022). Cleveland Clinic. https://my.clevelandclinic.org/health/articles/22611-epinephrine-adrenaline
[7]. https://www.facebook.com/verywell. (2021). What Is Adrenaline? Verywell Health. https://www.verywellhealth.com/what-is-adrenaline-5094550
[8]. Epinephrine inhalation aerosol | Cleveland Clinic. (2023). Cleveland Clinic. https://my.clevelandclinic.org/health/drugs/19722-epinephrine-inhalation-aerosol
[9]. Office, N. (2020). EPINEPHRINE | CAMEO Chemicals | NOAA. Noaa.gov. https://cameochemicals.noaa.gov/chemical/16207
[10]. PubChem. (2023). Hazardous Substances Data Bank (HSDB): 4289. @Pubchem; PubChem. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/4289
[11]. 2D Chemical Structure Image of (S)-adrenaline on Its Chemical Structure Page. (2022). Mol-Instincts. https://www.molinstincts.com/structure/S-adrenaline-cstr-CT1002341597.html #:~:text=The%20(S)%2Dadrenaline%20molecule,1%20secondary%20alcohol(s).
[12]. 2D Chemical Structure Image of Epinephrine on Its Chemical Structure Page. (2022). Mol-Instincts. https://www.molinstincts.com/structure/epinephrine-cstr-CT1002033985.html
[13]. Nutr, J. (n.d.). An Overview of Phenylalanine and Tyrosine Kinetics in Humans. National Library of Medicine. Retrieved March 26, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2268015/
[14]. Pharmacology Animation. (n.d.). YouTube [YouTube]. https://youtu.be/H1qUpuf0ZP4
[15]. Pelto, L., B.D Byylund, Salminen, S., & Isolauri, E. (n.d.). Epinephrine. ScienceDirect. Retrieved March 26, 2023, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/epinephrine
[16]. Sykora, M., Schönenberger, S. and Bösel, J. Sykora, M., Schönenberger, S., & Bösel, J. (2016). (n.d.). Norepinephrine. ScienceDirect. Retrieved March 26, 2023, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/
[17]. Wolter, L., & Dhaun, H. (2001). https://patentimages.storage.googleapis.com/03/d5/1a/04a1f 96cbe6f72/US6218575.pdf
[18]. Catecholamine blood test Information | Mount Sinai - New York. (2013). Mount Sinai Health System. https://www.mountsinai.org/health-library/tests/catecholamine-blood-test#:~:text= Normal%20Results,(764.3%20pmol%2FL).
[19]. Adrenaline. (2023, January 27). Healthdirect.gov.au; Healthdirect Australia. https://www.healthdirect.gov.au/adrenaline#:~:text=This%20is%20known%20as%20the,within%202%20or%203%20minutes.
[20]. Pelto, L., Salminen, S., & Isolauri, E. (2002). MILK ALLERGY. Encyclopedia of Dairy Sciences, 1821–1827. https://doi.org/10.1016/b0-12-227235-8/00306-0
[21]. Nall, R. (2018, February 15). What’s the Difference Between Epinephrine and Norepinephrine? Healthline; Healthline Media. https://www.healthline.com/health/epinephrine-vs-norepinephrine#uses
[22]. William_Bates. (2023). Bionity.com. https://www.bionity.com/en/encyclopedia/ William_Bates. html
[23]. The Use of Extract of Suprarenal Capsule in the Eye - www.Central-Fixation.com. (2023). Central-Fixation.com. https://www.central-fixation.com/bates-medical-articles/use-of-extract -of-suprarenal-capsule.php
[24]. Hassanpour, S. E., Zirakzadeh, H., & Aghajani, Y. (2020). The Effect of Subcutaneous Epinephrine Dosage on Blood Loss in Surgical Incisions. WORLD JOURNAL of PLASTIC SURGERY, 9(3), 309–312. https://doi.org/10.29252/wjps.9.3.309
[25]. Epinephrine Intramuscular: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD. (2023). Webmd.com. https://www.webmd.com/drugs/2/drug-93171/epinephrine- intramuscular/details#:~:text=This%20medication%20is%20used%20in,face%2C%20lips%2C%20and%20throat.
[26]. Trafton, A. (2020, April). Researchers achieve remote control of hormone release. MIT News | Massachusetts Institute of Technology. https://news.mit.edu/2020/remote-control-hormone-release-nanoparticles-0410
[27]. Fitzgerald, M. (2008, August 27). A Mixed Blessing for Memory: Stress and the Brain - Brain Connection. Brain Connection. https://brainconnection.brainhq.com/2008/08/26/a-mixed-blessing-for-memory-stress-and-the-brain/#:~:text=The%20limbic%20system%20includes%20the,been%20associated%20with%20improved%20memory.
[28]. Hypothalamic-Pituitary-Adrenal (HPA) Axis. (n.d.). https://www.unh.edu/ pacs/sites/default/files/media/2020-07/stress-and-your-body-handout.pdf









