1. Introduction
Major depressive disorder (MDD) is a specific clinical diagnosis of depression which poses a significant threat to mental health, affecting millions of individuals worldwide [1]. Patients with MDD are usually clinically characterized and diagnosed with depression, reticence, sleep disorders, and idiopathic pain, and those with severe depression may develop self-harm and suicide [2,3]. Additionally, MDD has many chronic comorbidities [4], which are linked with increased difficulty of treatments and increased health care utilization and costs. For these physical and psychological conditions, depressed patients usually have lower quality of life with higher morbidity and mortality [5]. However, the pathogenesis of depression is complex, and its mechanism is not yet well defined. Research shows that regional alterations often occur in brain volumes of MDD patients [6] and functional changes in cognitive control networks [7]. In contrast to healthy individuals, patients with MDD might experience monoamine deficiency [8], abnormal function of the hypothalamic-pituitary-adrenal (HPA) axis [9], dysregulation of inflammatory cytokine [10], lower brain-derived neurotrophic factor (BDNF) [11], poorer neuroplasticity [12], and the changes in the mitochondrial structure and function [13]. Moreover, the weak antioxidant capacity [14], dysregulation of the gut microbiota [15], overactivated kynurenine metabolic pathway and reduced tryptophan (TRP) [16] can also contribute to the pathological development in MDD patients.
Currently, the most prevalent treatment for depression is the use of synthetic medications, such as anti-depressants. According to Warren, the mechanisms behind these medications have not been fully elucidated [17]. However, there are different theories on the effects of anti-depressant medications. One theory, the monoamine theory, believes that anti-depressants target certain neurotransmitters, such as serotonin, noradrenaline and dopamine, to help with the chemical imbalances present within the brain [17-19]. According to the UK National Health Service, commonly taken anti-depressants include selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs) and noradrenaline and specific serotonergic antidepressants (NASSAs). Approximately 60% of patients worldwide exhibit an improvement in symptoms within two months of taking the above-mentioned medications [20]. However, some patients are irresponsive to anti-depressants, with 10%-30% of the patients showcasing zero to little improvements in MDD [21]. Other than the effectiveness among different patients being uncertain, side effects of anti-depressants are also universally seen occurring within patients. For example, patients prescribed paroxetine, an anti-depressant of the SSRI class, can experience constipation, blurred vision, dizziness and fatigue [22]. In contrast, an alternative treatment to depression is plant-based medicine (PBM). It is able to reproduce similar anti-depressant effects when tackling depression with fewer side effects compared to anti-depressant medications [23].
PBM is a term used to represent medicines from the earth's natural plant resources [24]. Human beings have relied on PBMs to maintain health and treat diseases for centuries, and in recent years, more and more researchers have combined traditional plants with modern medicines to achieve therapeutic effects [25, 26]. PBM has become an essential direction for exploring new antidepressant therapies because of their bioactive ingredients with potentially synergistic effects, less disruptive effects, and thus a lower risk of side effects [27]. Numerous studies suggest that plant extracts and their bioactive ingredients exhibit antidepressant activity through multiple mechanisms. This review discusses monoamine neurotransmitter regulation, neuroplasticity adjustment [28], HPA axis hormones adjustment, BDNF regulation [29], oxidative stress and mitochondrial dysfunction regulation, gut-brain microbiota regulation and raising mood from anti-inflammatory [30].
This review aims to illustrate and summarize various mechanisms whereby PBMs can have antidepressant effects, providing a reference for future studies. Plant products have been used to cure human diseases for centuries, and these products or analogues are still used to treat various diseases, either alone or as part of herbal compositions [31]. A straightforward and comprehensive understanding of the mechanisms of PBMs as a therapy offers a new orientation for depression research and treatment of patients.
2. Method
To collect enough studies of mechanisms of plant-based medicines in treating MDD, a comprehensive search was done on databases such as PubMed, Web of Science, Scopus, etc. The search keywords included depression, MDD, depression mechanisms, plant-based medicines, plant-based medicines antidepressant effect, plant-based medicines antidepressant mechanisms, etc. The cited articles cover the period from April 1996 to June 2024.
To select articles relevant to our objective from the records, the following criteria were used: studies that reported the traditional uses, antidepressant mechanisms, and bioactivities of PBMs; priority was given to literature published after 2020; priority was given to literature using clinical or animal models; and English abstracts were used for publications that were not written in English. Non-English studies and those not meeting the selection criteria will be eliminated.
3. Result
3.1. Monoamine neurotransmitter regulation
The monoamine hypothesis postulates that the underlying pathophysiological mechanism of MDD lies in reduced levels of monoamine neurotransmitters in the central nervous system, encompassing 5-hydroxytryptamine (5-HT), norepinephrine (NE) and dopamine (DA) [32]. Numerous studies have shown that 5-HT, NE and DA greatly influence brain regions involved in emotional processing and stress regulation [33]. Monoamine oxidases (MAOs), including two isozymes MAOA and MAOB, also play a crucial part in catalyzing the oxidation and, thus, degradation of neurotransmitter amines, promoting reactive oxygen species (ROS) production in mitochondria and regulating the transmission of both monoamine neurotransmitters and oxidative stress in the brain [34].
Many antidepressant treatments currently used are developed based on this theory, focusing on monoamine targets [35], especially MAOs. Increased oxidative and nitrosative stress constitutes a significant etiological factor in the pathogenesis of neurodegeneration and neuronal apoptosis, as well as the attenuation of neurogenesis and neuroplasticity [36, 37]. Therefore, MAOs have become the major target when designing inhibitors of neurodegenerative diseases, as the inhibition of MAOs restrains monoamine degradation, thus leading to an increase in the content of monoamines in the brain and ultimately affecting MDD. While these results are promising, recent evidence is also shedding light on potential limitations. The use of synthetic medications causes instantaneous changes in monoamine levels, their therapeutic effects are observed after weeks of treatment, and most of the used MAOs synthetic medicines cause long-term irreversible inactivation [38].
On the contrary, PBM may have a more temperate effect. Hemerocallis fluva L. is a perennial herb of the Hemerocallidoideae family commonly found in Asia [39]. Ancient traditional Chinese medicine literature indicates that its roots are beneficial for soothing the spirit and equilibrium of temperament, thereby alleviating melancholy. The most effective components closely related to antidepression in Hemerocallis fluva L. are kaempferol, anthraquinone and vanillic acid. Kaempferol, a type of flavonoid, has been demonstrated to possess potent inhibitory effects on MAOs and antioxidant and neuroprotective properties in mouse models [40]. Though human MAOs and mouse MAOs have sequence heterogeneity, studies have shown that kaempferol also works as an inhibitor of human MAOA [41]. Anthraquinone, an aromatic organic compound, and vanillic acid have proved to be potent inhibitors of human MAOs activity, resulting in the reduction of MAOA and MAOB levels and exerting an impact on MDD [42]. Also, plant polysaccharides are important bioactive components of PBM, and they have antioxidant, anti-inflammatory, antineoplastic, antidepression, and other biological activities [43]. Polygonati sibiricum is a traditional medicinal and edible plant used to treat MDD, and Polygonatum sibiricum polysaccharides (PSP) is one of its main active components. A study showed that PSP has an active antidepressant effect on lipopolysaccharide and chronic unpredictable mild stress-induced depression mice models by reversing the decline of 5-HT in hippocampal [44].
3.2. Hypothalamus-pituitary-adrenal axis regulation
As a major stress neuroendocrine system, the HPA axis significantly influences the pathological development of MDD. When somatic stimuli, such as hunger, inflammation, and perceived psychological stress, are received by corticotropin-releasing factor (CRF) neurons, the hypothalamus will respond by producing CRF and vasopressin (AVP). CRF and AVP will further stimulate the secretion of adrenocorticotrophic hormone (ACTH) in the pituitary gland, which contributes to increasing glucocorticoids and, more specifically, corticosterone (CORT) in rodents and cortisol in humans [9]. Numerous studies show that the main hallmark of depression is an excessive secretion of cortisol [45]. In the presence of chronic stress, prolonged glucocorticoid production binds to glucocorticoid receptors (GR), damaging the hippocampus and locus coeruleus, thus reducing GR availability and ultimately disrupting the HPA axis. This leads to cognitive decline, insomnia, and low emotional states, thus contributing to the pathogenesis of MDD [46].
Current antidepressants work by reducing the concentration of AVP, ACTH and CRF and promoting the concentration of GRs. PBM contains a variety of active ingredients that affect the HPA axis. Icariin is a flavonoid isolated from Epimedium brevicornum, which was found to inhibit the CRF elevation in behavioral despair model mice [47]. Additionally, various plant polysaccharides exhibit great potential in regulating the HPA. The PSP [44], Lycium barbarum polysaccharide (LBP) [48], Lily polysaccharides (LLP), and Astragalus polysaccharides (APS) were all observed to reduce serum CORT levels. Another kind of bioactive ingredient found in PBM is saponins. When CUMS rats were administrated with saponins from Panax ginseng, ACTH action in the adrenal gland was inhibited, and the modulation of HPA function appeared to cause an anti-depressed-like effect [49, 50]. What is more, piperine is reported to reduce ACTH and CRF in serum, improving the behavioral disorder in rats [51].
In summary, these studies support the conclusion that HPA axis dysfunction is related to the abnormal concentration of CRF, ACTH, and CORT, and numerous active principles found in PBMs may regulate this pathway by adjusting its hormones via multiple mechanisms. Thus, paying more attention to the specific PBM and launching further research to extract novel components and biochemicals could provide novel treatment options for depression patients.
3.3. Regulation of immune dysregulation and inflammatory responses
Another mechanism of the antidepressant effect is the regulation of dysregulation in the cellular immune of patients, reducing the release of pro-inflammatory cytokines, such as interleukin (IL)-1b, IL-6 and tumour necrosis factor (TNF)-a [52]. These cytokines impair the normal function of neurons and affect the transmission of neurotransmitters. Additionally, depression patients have shown higher levels of leukocytes, which may also indicate the presence of an inflammatory process [53]. So, PBM ingredients with anti-inflammatory effects potentially show antidepressant function; specific pathways are described in the following paragraph.
Several ingredients from PBM are reported to have antidepressant effects through inflammation regulation mechanisms. Icariin has shown an antidepressant effect in rat models by inhibiting the nod-like receptor protein 3 (NLRP3) - inflammasome/caspase-1/IL-1b axis and nuclear factor kappa B (NF-κB) signalling activation, which furtherly causes increased antioxidant levels and anti-inflammatory effects on brain tissue [54]. Icariin can be extracted in plants from Epimedium. Senegenin, a bioactive ingredient in Polygala tenuifolia Willd, has also exerted an antidepressant effect in chronic unpredictable mild stress (CUMS) induced mice [55]. Senegenin significantly improved the behavioural abnormalities of mice and increased the behavioural score of mice in the Sucrose preference test (SPT) by inhibiting the protein activation associated with the NLRP3 inflammasome pathway and IL-1b secretion [55]. Another antidepressant ingredient is Vanillic acid, which originated from Angelica sinensis (Oliv.) Diels. Singh et al. (2015) [56] studied anti-inflammatory effect of Vanillic acid in mice model, they observed a reduction of TNF-a and acetylcholinesterase (AChE), and a increase in antioxidants in mice after Vanillic acid treatment. This result revealed the anti-inflammatory effect of Vanillic acid and its antidepressant potential. In addition, Ginseng total saponins (GTS), major bioactive components of Panax ginseng C.A. Meyer, also have an antidepressant effect. Chronic mild stress (CMS) rats had higher sucrose preference index, locomotor activity, and lower latency of feeding in novel environments after GTS treatment [57]. Moreover, 7-day treatments of GTS significantly shortened the immobility time in the forced swimming test (FST) in rats [57]. The potential mechanism is the decreased mRNA expression of IL-1b, IL-6, TNF-a, and indoleamine 2,3-dioxygenase (IDO) in the hippocampus, which ameliorates inflammation and further maintains normal neurotransmission [58].
Studies that associate depression with immunological and inflammatory changes have a long history, which can be traced back to the birth of psychoneuroimmunology in 1975, and new findings continue to appear in the 2020s [59]. The mechanism of the antidepressant effect of PBM will continue to be explored as the interaction between depression and inflammation becomes clearer.
3.4. Brain-derived neurotrophic factor regulation
BDNF is one of the neurotrophic factors (NTFs), which are proteins in the central nervous system (CNS) responsible for the growth and maintenance of neurons [60]. BDNF has numerous functions, such as synaptic plasticity [61], regulation of mood and depression [62], and new neuron differentiation and growth [63]. This protein is distributed in the brain, specifically in the hippocampus, basal forebrain, olfactory bulb, and cerebral cortex [61]. BDNF carries out its functions by binding and activating the Tropomyosin receptor kinase (Trk) B, which is part of the larger Trk receptors, and the p75 neurotrophin receptor (NTR) [29]. Therefore, BDNF promotes neuroplasticity and neuronal survival by activating intracellular signalling pathways [64]. Studies have shown the connection between psychiatric illnesses (such as depression and schizophrenia) and the alterations in BDNF expressions [65]. This is supported by numerous meta-analyses [66] suggesting that patients with depression tend to have lower BDNF plasma levels in contrast to a healthy person [67].
Several PBMs have been reported to increase the levels of BDNF, helping with the treatment of depression. One example is Hemerocallis citrina, which is commonly used in Chinese medicine for mood disorders and depression [68]. They produce anti-depressant effects and improve the functioning of the neurotrophin system [69]. Experiments with a depression rodent model treated with Hemerocallis citrina ethanolic extracts (HCE), undergoing the sucrose preference test (SPT), showed increased contents of BDNF in the frontal cortex and hippocampus [69]. Another example is Gladiolus Dalenii, which is typically used in the west as a cure for central nervous disorders such as schizophrenia and depression [70]. Experimental results done on rat models with epilepsy-associated depression showcased increased BDNF levels in the hippocampus [71]. Degraded Porphyra are bioactive polysaccharides originating from Porphyra Haitanensis and exhibit anti-depressant effects when treating depression-like animal models [72]. Based on experimentations done by Yi [72], results have shown that degraded Porphyra treatment helps activate the BDNF signalling pathway in mice models. Scutellaria baicalensis is widely used in China as herbal medicine and primarily, patients are prescribed the plant’s dry roots as a treatment for depression [73]. The main components, baicalin and baicalein, have been shown to increase monoamine transmitter brain neurotrophic factor levels [74]. Albizia julibrissin is used in clinical practices, exerting anti-depressant responses from animal models [75]. According to Huang [75], flavonoids extracted from Albizia julibrissin have demonstrated increased expression of BDNF levels and its receptor, TrkB, in the hippocampus.
3.5. Oxidative stress and mitochondria dysfunction regulation
Oxidative stress occurs when the normal balance between the production of free radicals (particularly ROS) and antioxidant defences is disrupted. The overwhelming ROS and reduced antioxidants are mainly characterized by pathological conditions caused by oxidative stress [76]. These extra ROS are produced by long-term exposure to environmental factors, like smoking and ultraviolet UV radiation [77], and physical or psychological stress [78]. Depression patients are always accompanied by lower antioxidant intake (such as Vitamin A, C, E, superoxide dismutase) and glutathione peroxidase (GPx), which greatly influences the pathogenesis and progression of depression. Additionally, some studies show that oxidative stress also links with the stress response, neurogenesis, synaptic plasticity’s imbalance present, neuroinflammation, serotonergic pathways, and HPA axis [79]. When the excessive oxidative stress presence, all these factors are intensified [80].
Based on the overall correlation between the various pathways of depression, PBMs show antidepressant effect by connecting oxidative stress with other mechanisms, such as increasing the level of 5-TH [81], improving the negative feedback of HPA [82], reducing the level of inflammation [83], and increasing the level of BDNF, affecting neuroplasticity in the end [84]. For instance, Centella asiatica, a herbaceous species, employed in clinical and cosmetic treatments for its ability in boosting memory, improve brain function and prevent cognitive deficits. Its main bioactive constituents are Centalla asiatic triterpenic acid (CATA), asiatic acid (AA) and madecassic acid (MA), which proved to have not only anti-oxidative effect, but also neuroprotective, anti-inflammatory, anti-allergic and anti-depressant function [85]. Beyond that, Ginsenoside Rg3 [86], naringenin [87], Polygala japonica [88], and silymarin [89] may also improve depression-like behaviors by the anti-oxidative action. Geniposide [90] and saikosaponin [91] improve the negative feedback of the HPA axis in the process of anti-oxidation. Meanwhile, EGb761 [92], an extract of ginkgo biloba leaves, which mainly includes quercetin, kaempferol and isorhamnetin, can reduce oxidative stress and thus ameliorate lipopolysaccharides-induced depression-like behaviors, resulting the decrease of inflammation and CORT release at the same time. The ROS production mainly happens in the cell mitochondria. Mitochondrial dysfunction is observed in various brain regions of many MDD patients. Preclinical and clinical research indicates that increased oxidative stress is the main reason for mitochondria dysfunction in depressed individuals [93]. Additionally, the interaction between oxidative stress and mitochondrial dysfunction in patients' brains causes a continuous vicious cycle.
3.6. Gut microbiota regulation
PBM can also exert its antidepressant effect by regulating gut microbiota. The mechanism of this regulation is the microbiota–gut–brain axis (MGBA) signalling pathway, which impacts mood and cognitive performance through immunological-, metabolic-, neurological-, and hormonal-mediated approaches [94]. For instance, gut microbiota contributes to the gut homeostasis through direct interacting with the intestinal epithelial barrier (IEB), and sysbiosis of gut microbiota induces depression-like behavior in rats [94]. MGBA connects gut microbiota and depression, and more and more evidence are supporting their association [95]. The following paragraph demonstrates several mechanisms of action on the MGBA by PBMs.
Xiaoyaosan (XYS), a PBM consisting of eight Chinese herbs, including Radix Angelicae Sinensis and Rhizoma Zingiberis officinalis recens, had an antidepressant effect in CUMS rats by reducing the abundance of Desulfovibrio, thus decreasing lipopolysaccharide, inflammation, and depression [96]. Chaihu-Shugan-San (CSS), another PBM composed of seven Chinese herbs, showed depression alleviating function in mice models through regulation of gut microbiota by reducing NF-κB-mediated BDNF expression [97]. Other than these herbal compounds, some single Chinese herbs have antidepressant effects as well. Bupleurum chinense is a single Chinese herbal medicine derived from the roots of the Bupleurum plant [98]. Studies have indicated that Bupleurum chinense had an antidepressant effect in mice by regulating the composition of intestinal flora [99]. Turmeric, where curcumin is the main bioactive ingredient, also decreased depressive-like behaviors of mice, through regulation of gut microbiota with the involvement of supraspinal serotonergic system and downstream gamma-aminobutyric acid (GABA)A receptor [100]. PBMs like single Chinese herbs and Chinese herbal compounds have proved beneficial in treating depression in rats, providing evidence for future studies of depression therapy [94].
3.7. Neuroplasticity
Neuroplasticity, or neural plasticity, is the ability of the brain to develop new neuronal connections in response to changes in the body and environment [101]. There are three aspects of neuroplasticity: synaptic plasticity, structural plasticity, and functional plasticity [102]. According to Abbott & Nelson, synaptic plasticity refers to the changes taking place in synapses, which are junctions between neurons where communication occurs [103]. Structural plasticity is the physical changes occurring in the structure of the neurons and their networks in order to adapt to intrinsic and extrinsic factors [104]. Functional plasticity is the brain's ability to move the functions of a damaged area into a non-damaged area, typically in response to an injury [105].
Neuroplasticity occurs primarily in two regions within the brain: the hippocampus, responsible for memory and learning [106], and the prefrontal cortex, essential for attention and memory regulation [107,108]. In the hippocampus, it has been demonstrated that decreased hippocampal volume is associated with MDD [109]. One possible explanation for that is that the damage done to the hippocampus is due to the boosting of glucocorticoids (steroid hormones) during depressive episodes [110]. Mood changes and memory loss often occur in depressed patients [111] as the hippocampus plays a vital role in memory and learning [106]. On the other hand, volume reduction in the prefrontal cortex occurs due to the disruption of neurons and their networks in depression [112]. A reduction in glutamate metabolism in the GABAergic pathway was demonstrated in mouse models with stress-induced depression [113].
A PBM that enhances neuroplasticity is Lycium barbarum, which is widely used in Asian countries. Its effectiveness has been found in the hippocampus of rats, reducing depression-like symptoms by enhancing synaptic plasticity [114]. Although there is a lot of research done on the mechanism of neuroplasticity, more research needs to be done due to the lack of PBMs associated with neuroplasticity to help patients have a better understanding of the benefits of PBMs in treating major depressive disorder. A summary of mechanisms is shown in Table 1.
Table 1: PBMs and corresponding mechanisms of antidepressant effect
PBM | Source | Mechanism | |
Anthraquinone | Hemerocallis fluva L. | Monoamine neurotransmitter regulation | [42] |
Kaempferol | Hemerocallis fluva L. | Monoamine neurotransmitter regulation | [40] |
Icariin | Epimedium brevicornum | HPA axis regulation | [47] |
Inflammation regulation | [54] | ||
PSP | Polygonatum sibiricum | Monoamine neurotransmitter regulation | [44] |
HPA axis regulation | [44] | ||
LBP | Lycium barbarum | HPA axis regulation | [48] |
Neuroplasticity | [114] | ||
LLP | Lily | HPA axis regulation | [67] |
APS | Astragalus | HPA axis regulation | [67] |
Saponins | Panax ginseng | HPA axis regulation | [49] [50] |
Inflammation regulation | [57] | ||
Vanillic acid | Angelica sinensis | Inflammation regulation | [56] |
Monoamine neurotransmitter regulation | [42] | ||
Senegenin | Polygala tenuifolia | Inflammation regulation | [55] |
Centella asiatica | Centella asiatica | Oxidative Stress regulation | [85] |
EGb761 | Ginkgo biloba | Oxidative Stress regulation | [92] |
XYS | PBM compound | Gut microbiota regulation | [96] |
CSS | PBM compound | Gut microbiota regulation | [97] |
Bupleurum chinense | Bupleurum plant | Gut microbiota regulation | [99] |
curcumin | Turmeric | Gut microbiota regulation | [100] |
piperine | piper nigrum L | HPA axis regulation | [51] |
Hemerocallis crtrina | Hemerocallis crtrina | BDNF regulation | [115] |
Gladiolus Dalenii | Gladiolus Dalenii | BDNF regulation | [71] |
Porphyra Haitanensis | Porphyra Haitanensis | BDNF regulation | [72] |
Scutellaria baicalensis | Scutellaria baicalensis | BDNF regulation | [116] |
Albizia julibrissin | Albizia julibrissin | BDNF regulation | [75] |
4. Discussion
In the anti-depression actions of PBMs, serotonergic, dopaminergic, and noradrenergic systems play an important role. PBMs' monoamine control mechanism is primarily influenced by the suppression of the monoamine oxidase response and increased synaptic monoamine availability. The melioration effect of PBMs is intimately connected to the normalization of HPA axis dysfunction, as the HPA axis plays a critical role in the pathogenesis of depression. Besides, PBMs that can reestablish neural plasticity, increase BDNF, and reduce nerve harm caused by negative stimuli are more likely to help people recover from depression since impaired neuroplasticity and reduced BDNF is a critical aspect of the brain in response to depression. Additionally, it is well acknowledged that inflammation and reduced immunity, which are common processes in many illnesses, play an important role in the development of depressive disorders, so anti-inflammatory PBMs have the potential to treat MDD. Last but not least, Some PBMs can alleviate depressant symptoms by regulating gut microbiota or mitochondria dysfunction. Multiple studies have shown that PBMs have many advantages, including comprehensive disease intervention [117] and alleviation of side effects caused by single-link action [118], so PBMs have the capacity and potential to work synergistically in the treatment of depression. For example, Hemerocallis can treat MDD through neurotransmitter regulation and BDNF modulate, and PSP regulate HPA axis and monoamine neurotransmitter to have antidepressant effects. Another sample, Schizandra chinensis (Turcz.) Baill., which has many active ingredients, including schizandrin A (Sch A), schizandrin B (Sch B), schizandrin C (Sch C), schisantherin A (STA), schisandrin (SCH), α-isocubebenol (ICO), gomisin A and polysaccharides, can treat MDD through antioxidative effect, regulation of monoamine neurotransmitters and modulation of BNDF [119-121]. A study also found that Sch A, a predominant ingredient of lignans in Schizandra chinensis, exhibits a pronounced antidepressant effect on lipopolysaccharide induced depression in mice models by modulating intestinal microbiota and suppressing TLR4/NF-κB signal pathways in the hippocampus, thereby diminishing neuroinflammation [122]. Rutin, a mainly flavonoid quercetin found in Schizandra chinensis fruit, was proved to have an effect on neurodegenerative disorders including MDD, Alzheimer’s disease, and Parkinson’s disease [123]. Based on the samples discussed above, we can see that PBM treatments for MDD are collaborative (Fig. 1).
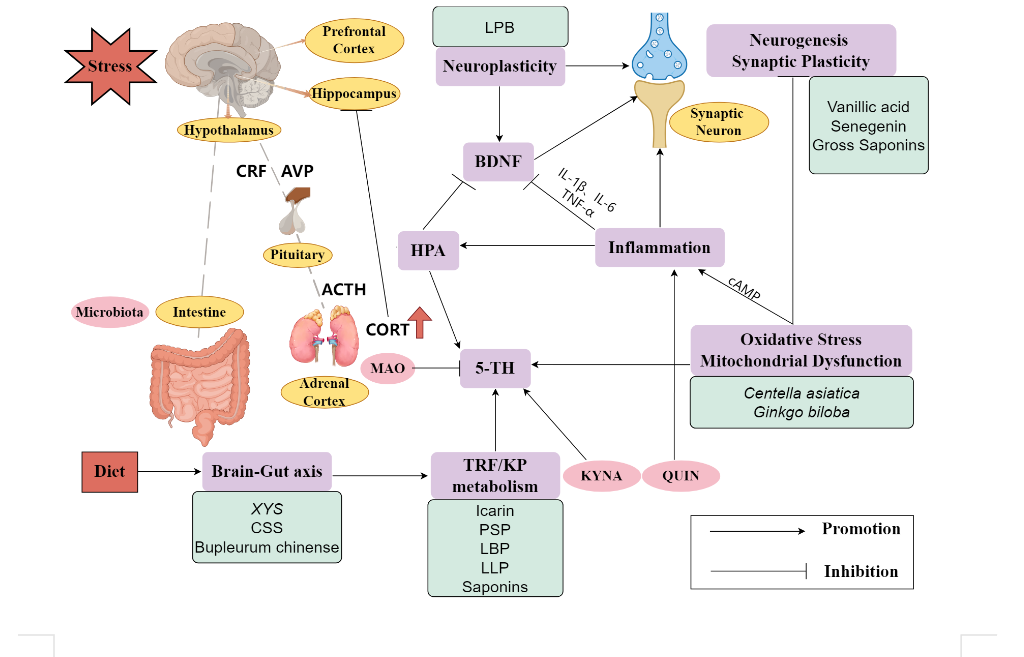
Figure 1: The mechanisms of PBMs and the interaction between them. The internal and chronic stress and diet stimulate the main axis, like the HPA cortex axis and brain-gut axis, causing some domain neurotransmitters (ACTH, CORT) to increase and disorder in tryptophan metabolism (TRF) /kynurenine pathway (KP). Additionally, the metabolic products kynurenic acid (KYNA) (neuroprotective) and quinolinic acid (QUIN) (neurotoxic) are accumulated at different levels (QUIN is more than KYNA), affecting other mechanisms like monoamine (5-TH) and neuroinflammation. PBMs function with an antidepressant effect by inhibiting these pathways
Through the results, it is clear that PBM can work on multiple targets, which significantly improves the symptoms of MDD compared with a single antidepressant. Except for collaborative and multi-targeted effects, PBM shows great potential in other chronic diseases and complications, indicating it can be used for personalized treatment in specific patients. The current synthetic antidepressants are often accompanied by certain physiological side effects, including dizziness, insomnia, and dry mouth. PBM, as an internal regulatory agent, can significantly alleviate these symptoms, thereby achieving a more comprehensive and effective antidepressant outcome. It also provides a gentle alternative for individuals who are allergic to synthetic antidepressants.
There are various limitations found in both PBMs and the mouse model behavioural studies used to test the mechanisms of PBMs. One limitation of PBMs is the need for more standardisation and their likeability to be affected by various factors such as medicine cultivation, geographical location and seasonal harvest [124]. Since only active constituents are extracted from plant sources to make up for the PBMs taken by patients, it is important to standardise them to ensure the quality of the raw materials used [125]. Subsequently, PBMs need to be taken longer than anti-depressants to be effective and exert anti-depressant effects. An example is St. John's Wort, which needs to be taken between 2-6 weeks to have therapeutic effects. Furthermore, access to PBMs still needs to be improved for a certain audience due to the higher cost if taken long-term. And healthcare and insurance typically only covers the expenses of anti-depressants as medication for MDD, there is a difficulty for patients to pruchase PBMs. Mouse models are frequently used in behavioural studies, especially in psychiatric disorders such as depression, to test the effectiveness and safety of bioactive compounds in clinical trials [126]. However, the limitations and shortcomings are constantly questioned due to the reliability of the results and the ethical concerns about using animals as subjects of research [127]. The poor reproducibility of the mice model results in humans [128] is supported by researchers finding a weak correlation between humans and mice in a study comparing their immune responses [129]. Moreover, the lack of reliability of the data can be due to the variations in the experimental laboratory of the mouse and the genetic differences occurring within the genetically modified mice [128]. On the other hand, the use of animal models raises ethical issues with little amount experiments violating the 3Rs, neglecting proper treatments on experimental animals [130]. According to the National Health and Medical Research Council (2019), the 3Rs stand for replacement, reduction and refinement, accounting for the care of animals.
Given the above limitations, future research could follow the following directions as improvements. First, long-term double-blind, clinical, if possible, experimental studies should be carried out to study the antidepressant effect, efficacy, and safety of PBM on patients. And some of the trials using mice model should be ethically redesigned to follow the 3Rs principle. In addition, it is significant to deepen the research on the synergistic effects of different PBMs, furtherly demonstrating their interactive mechanism. Multiomics technology has been reported to be beneficial in studies of TCM mechanisms [98], which could be an inspiration for similar research on PBMs. What is more, PBMs usually aim at multiple targets and exert comprehensive effects in the treatment of depression. Thus, an exact identification of active ingredients and a standardization of chemical composition are needed to benefit industrial production and clinical application.
5. Conclusion
This review illustrated and summarized seven different mechanisms of antidepressant effect from the perspective of PBMs, aiming to provide an integral guide for future studies. After analyzing the literature, this review found that these mechanisms showed their own characteristics and somehow interacted with each other, exerting the antidepressant effect holistically. Some limitations of studies in this area also needed to be addressed, which could be further research goals. Only when the efficacy and safety of PBM are fully confirmed can it be popularized in the market and help patients with depression.
Acknowledgment
The authors would like to thank professor Gioia Polidori who patiently provided helpful comments for revising the paper.
References
[1]. Nabavi, S. M., Daglia, M., Braidy, N., & Nabavi, S. F. (2017). Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutritional neuroscience, 20(3), 180–194.
[2]. Stockmeier, C. A., & Rajkowska, G. (2004). Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues in clinical neuroscience, 6(2), 185–197.
[3]. Kvam, A. K., & Waage, A. (2015). Health-related quality of life in patients with multiple myeloma--does it matter?. Haematologica, 100(6), 704–705.
[4]. Marx, W., Penninx, B. W. J. H., Solmi, M., Furukawa, T. A., Firth, J., Carvalho, A. F., & Berk, M. (2023). Major depressive disorder. Nature reviews. Disease primers, 9(1), 44.
[5]. Elderon, L., & Whooley, M. A. (2013). Depression and cardiovascular disease. Progress in cardiovascular diseases, 55(6), 511–523.
[6]. Zhou, Q. G., Zhu, X. H., Nemes, A. D., & Zhu, D. Y. (2018). Neuronal nitric oxide synthase and affective disorders. IBRO reports, 5, 116–132.
[7]. Scheepens, D. S., van Waarde, J. A., Ten Doesschate, F., Westra, M., Kroes, M. C. W., Schene, A. H., Bockting, C. L. H., Schoevers, R. A., Denys, D. A. J. P., Ruhé, H. G., & van Wingen, G. A. (2020). Effectiveness of Emotional Memory Reactivation vs Control Memory Reactivation Before Electroconvulsive Therapy in Adult Patients With Depressive Disorder: A Randomized Clinical Trial. JAMA network open, 3(8), e2012389.
[8]. Charney D. S. (1998). Monoamine dysfunction and the pathophysiology and treatment of depression. The Journal of clinical psychiatry, 59 Suppl 14, 11–14.
[9]. Pariante, C. M., & Lightman, S. L. (2008). The HPA axis in major depression: classical theories and new developments. Trends in neurosciences, 31(9), 464–468.
[10]. Zunszain, P. A., Anacker, C., Cattaneo, A., Carvalho, L. A., & Pariante, C. M. (2011). Glucocorticoids, cytokines and brain abnormalities in depression. Progress in neuro-psychopharmacology & biological psychiatry, 35(3), 722–729.
[11]. Molendijk, M. L., Spinhoven, P., Polak, M., Bus, B. A., Penninx, B. W., & Elzinga, B. M. (2014). Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular psychiatry, 19(7), 791–800.
[12]. Duman, R. S., Aghajanian, G. K., Sanacora, G., & Krystal, J. H. (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature medicine, 22(3), 238–249.
[13]. Chen, H., Lu, M., Lyu, Q., Shi, L., Zhou, C., Li, M., ... & Ren, L. (2024). Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomedicine & Pharmacotherapy, 175, 116656.
[14]. Ana, R. D., Gliszczyńska, A., Sanchez-Lopez, E., Garcia, M. L., Krambeck, K., Kovacevic, A., & Souto, E. B. (2023). Precision Medicines for Retinal Lipid Metabolism-Related Pathologies. Journal of personalized medicine, 13(4), 635.
[15]. Hui, Y., Vestergaard, G., Deng, L., Kot, W. P., Thymann, T., Brunse, A., & Nielsen, D. S. (2022). Donor-dependent fecal microbiota transplantation efficacy against necrotizing enterocolitis in preterm pigs. NPJ biofilms and microbiomes, 8(1), 48.
[16]. Gong, X., Chang, R., Zou, J., Tan, S., & Huang, Z. (2022). The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Reviews in the neurosciences, 34(3), 313–324.
[17]. Warren, J. B. (2020). The trouble with antidepressants: why the evidence overplays benefits and underplays risks—an essay by John B Warren. bmj, 370.
[18]. Taylor, C., Fricker, A. D., Devi, L. A., & Gomes, I. (2005). Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cellular Signalling, 17(5), 549–557.
[19]. Delgado, P. (2024). Depression: the case for a monoamine deficiency. The Journal of Clinical Psychiatry, 61 Suppl 6.
[20]. Boseley, S. (2018). The drugs do work: antidepressants are effective, study shows. The Guardian, 21, 2018.
[21]. Al-Harbi, K. S. (2012). Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Preference and Adherence, 369–369.
[22]. Westenberg, H. G. M., & Sandner, C. (2006). Tolerability and safety of fluvoxamine and other antidepressants. International Journal of Clinical Practice, 60(4), 482–491.
[23]. Peng, S., Zhou, Y., Lu, M., & Wang, Q. (2022). Review of herbal medicines for the treatment of depression. Natural Product Communications, 17(11), 1934578X221139082.
[24]. van Wyk, A. S., & Prinsloo, G. (2020). Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. South African Journal of Botany, 133, 54-62.
[25]. Briskin, D. P. (2000). Medicinal Plants and Phytomedicines. Linking Plant Biochemistry and Physiology to Human Health. Plant Physiology, 124(2), 507-514.
[26]. Khan, H. (2014). Medicinal plants in light of history: recognized therapeutic modality. Journal of evidence-based complementary & alternative medicine, 19(3), 216-219.
[27]. Patwardhan, B., Vaidya, A.D., Chorghade, M.S., & Joshi, S.P. (2008). Reverse Pharmacology and Systems Approaches for Drug Discovery and Development. Current Bioactive Compounds, 4, 201-212.
[28]. Hirshler, Y., & Doron, R. (2017). Neuroplasticity-related mechanisms underlying the antidepressant-like effects of traditional herbal medicines. Eur Neuropsychopharmacol, 27(10), 945-958.
[29]. Colucci-D'Amato, L., Speranza, L., & Volpicelli, F. (2020). Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. International Journal of Molecular Sciences, 21(20), Article 7777.
[30]. Fries, G. R., Saldana, V. A., Finnstein, J., & Rein, T. (2023). Molecular pathways of major depressive disorder converge on the synapse. Molecular Psychiatry, 28(1), 284-297.
[31]. Najmi, A., Javed, S. A., Al Bratty, M., & Alhazmi, H. A. (2022). Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules, 27(2), 349.
[32]. Hasler, G. (2010). Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry, 9(3), 155-161.
[33]. Hamon, M., & Blier, P. (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry, 45, 54-63.
[34]. Edmondson, D. E. (2014). Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications [Article]. Current Pharmaceutical Design, 20(2), 155-160.
[35]. Gonda, X., Dome, P., Neill, J. C., & Tarazi, F. I. (2023). Novel antidepressant drugs: Beyond monoamine targets. CNS Spectr, 28(1), 6-15.
[36]. Leonard, B., & Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression [Review]. Neuroscience and Biobehavioral Reviews, 36(2), 764-785.
[37]. Maes, M., Kubera, M., Obuchowiczwa, E., Goehler, L., & Brzeszcz, J. (2011). Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways [Review]. Neuroendocrinology Letters, 32(1), 7-24.
[38]. Jiang, Y., Zou, D., Li, Y. M., Gu, S. M., Dong, J., Ma, X. J., Xu, S. J., Wang, F. S., & Huang, J. H. (2022). Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals, 15(10), Article 1203.
[39]. Li, J. (2007). Flora of China. Harvard Papers in Botany, 13(2), 301-302, 302.
[40]. Sloley, B. D., Urichuk, L. J., Morley, P., Durkin, J., Shan, J. J., Pang, P. K., & Coutts, R. T. (2000). Identification of kaempferol as a monoamine oxidase inhibitor and potential Neuroprotectant in extracts of Ginkgo biloba leaves. J Pharm Pharmacol, 52(4), 451-459.
[41]. Gidaro, M. C., Astorino, C., Petzer, A., Carradori, S., Alcaro, F., Costa, G., Artese, A., Rafele, G., Russo, F. M., Petzer, J. P., & Alcaro, S. (2016). Kaempferol as Selective Human MAO-A Inhibitor: Analytical Detection in Calabrian Red Wines, Biological and Molecular Modeling Studies. J Agric Food Chem, 64(6), 1394-1400.
[42]. Lin, H.-Y., Tsai, J.-C., Wu, L.-Y., & Peng, W.-H. (2020). Reveals of New Candidate Active Components in Hemerocallis Radix and Its Anti-Depression Action of Mechanism Based on Network Pharmacology Approach. International Journal of Molecular Sciences, 21(5), 1868.
[43]. Yan, T., Nian, T., Liao, Z., Xiao, F., Wu, B., Bi, K., He, B., & Jia, Y. (2020). Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int J Biol Macromol, 144, 427-440.
[44]. Shen, F., Song, Z., Xie, P., Li, L., Wang, B., Peng, D., & Zhu, G. (2021). Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. Journal of ethnopharmacology, 275, 114164.
[45]. Vreeburg, S. A., Hoogendijk, W. J., van Pelt, J., Derijk, R. H., Verhagen, J. C., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of general psychiatry, 66(6), 617–626.
[46]. Ancelin, M. L., Scali, J., Norton, J., Ritchie, K., Dupuy, A. M., Chaudieu, I., & Ryan, J. (2017). Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology, 77, 90–94.
[47]. Pan, Y., Kong, L. D., Li, Y. C., Xia, X., Kung, H. F., & Jiang, F. X. (2007). Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacology, biochemistry, and behavior, 87(1), 130–140.
[48]. Po, K. K., Leung, J. W., Chan, J. N., Fung, T. K., Sánchez-Vidaña, D. I., Sin, E. L., So, K. F., Lau, B. W., & Siu, A. M. (2017). Protective effect of Lycium Barbarum polysaccharides on dextromethorphan-induced mood impairment and neurogenesis suppression. Brain research bulletin, 134, 10–17.
[49]. Kim, D. H., Moon, Y. S., Jung, J. S., Min, S. K., Son, B. K., Suh, H. W., & Song, D. K. (2003). Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neuroscience letters, 343(1), 62–66.
[50]. Chen, Y., Han, T., Rui, Y., Yin, M., Qin, L., & Zheng, H. (2005). Effects of total triterpenes of Centella asiatica on the corticosterone levels in serum and contents of monoamine in depression rat brain. Zhong yao cai= Zhongyaocai= Journal of Chinese medicinal materials, 28(6), 492-496.
[51]. Hu, Y., Liao, H. B., Liu, P., Guo, D. H., & Wang, Y. Y. (2009). Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine, 7(7), 667–670.
[52]. Hayley, S., Hakim, A. M., & Albert, P. R. (2021). Depression, dementia and immune dysregulation. Brain, 144(3), 746-760.
[53]. Milaneschi, Y., Kappelmann, N., Ye, Z., Lamers, F., Moser, S., Jones, P. B., ... & Khandaker, G. M. (2021). Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Molecular psychiatry, 26(12), 7393-7402.
[54]. Liu, B., Xu, C., Wu, X., Liu, F., Du, Y., Sun, J., ... & Dong, J. (2015). Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience, 294, 193-205.
[55]. Li, H., Lin, S., Qin, T., Li, H., Ma, Z., & Ma, S. (2017). Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-κB regulating NLRP3 signal pathway. International immunopharmacology, 53, 24-32.
[56]. Singh, J. C. H., Kakalij, R. M., Kshirsagar, R. P., Kumar, B. H., Komakula, S. S. B., & Diwan, P. V. (2015). Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharmaceutical biology, 53(5), 630-636.
[57]. Dang, H., Chen, Y., Liu, X., Wang, Q., Wang, L., Jia, W., & Wang, Y. (2009). Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33(8), 1417-1424.
[58]. Kang, A., Hao, H., Zheng, X., Liang, Y., Xie, Y., Xie, T., ... & Wang, G. (2011). Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. Journal of neuroinflammation, 8, 1-14.
[59]. Beurel, E., Toups, M., & Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron, 107(2), 234-256.
[60]. Ramaswamy, S., & Kordower, J. H. (2008). Gene and Cellular Transplantation Therapies for Huntington's Disease. In CNS Regeneration (pp. 267-294). Academic Press.
[61]. Bathina, S., & Das, U. N. (2015). Brain-derived neurotrophic factor and its clinical implications. Archives of Medical Science, 6, 1164–1178.
[62]. Azman, K. F., & Zakaria, R. (2022). Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. International Journal of Molecular Sciences, 23(12), 6827–6827.
[63]. Lambert, W. M., Xu, C.-F., Neubert, T., Chao, M., Garabedian, M., & Jeanneteau, F. (2013). Brain-Derived Neurotrophic Factor Signaling Rewrites the Glucocorticoid Transcriptome via Glucocorticoid Receptor Phosphorylation. Molecular and Cellular Biology, 33(18), 3700–3714.
[64]. Correia, A. S., Cardoso, A., & Vale, N. (2023). BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics, 15(8), 2081–2081.
[65]. Sangiovanni, E., Brivio, P., Dell’Agli, M., & Calabrese, F. (2017). Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plasticity, 2017, 1–19.
[66]. Brunoni, A. R., Lopes, M., & Fregni, F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology, 11(8), 1169–1180.
[67]. Liu, X., Li, P., Ma, X., Zhang, J., Sun, X., Luo, X., & Zhang, Y. (2022). Association between plasma levels of BDNF and GDNF and the diagnosis, treatment response in first-episode MDD. Journal of Affective Disorders, 315, 190–197.
[68]. Hsu, Y.-W., Tsai, C.-F., Chen, W.-K., Ho, Y.-C., & Lu, F.-J. (2011). Determination of lutein and zeaxanthin and antioxidant capacity of supercritical carbon dioxide extract from daylily (Hemerocallis disticha). Food Chemistry, 129(4), 1813–1818.
[69]. Liu, X.-L., Luo, L., Liu, B.-B., Li, J., Geng, D., Liu, Q., & Yi, L.-T. (2014). Ethanol extracts from Hemerocallis citrina attenuate the upregulation of proinflammatory cytokines and indoleamine 2,3-dioxygenase in rats. Journal of Ethnopharmacology, 153(2), 484–490.
[70]. Fotsing, D., Ngoupaye, G. T., Ouafo, A. C., Stephanie, Kenneth, Y. A., & Elisabeth Ngo Bum. (2017). Effects of Gladiolus dalenii on the Stress-Induced Behavioral, Neurochemical, and Reproductive Changes in Rats. Frontiers in Pharmacology, 8.
[71]. Ngoupaye, G. T., Bum, E. N., & Mark, W. (2013). Antidepressant-like effects of the aqueous macerate of the bulb of Gladiolus dalenii Van Geel (Iridaceae) in a rat model of epilepsy-associated depression. BMC Complementary and Alternative Medicine, 13(1).
[72]. Yi, L., Zhang, M., Cheng, J., Wan, H., Li, C., Zhu, J., Zhang, Q., Liu, Q., & Xu, G. (2021). Antidepressant‐like Effects of Degraded Porphyran Isolated from Porphyra haitanensis. Molecular Nutrition & Food Research, 65(9).
[73]. Lee, H. W., Ryu, H. W., Kang, M.-G., Park, D., Lee, H., Shin, H. M., Oh, S.-R., & Kim, H. (2017). Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. International Journal of Biological Macromolecules, 97, 598–605.
[74]. Yu, H., Yin, Z., Yang, S., Ma, S., & Qu, R. (2015). Baicalin Reverses Depressive‐Like Behaviours and Regulates Apoptotic Signalling Induced by Olfactory Bulbectomy. Phytotherapy Research, 30(3), 469–475.
[75]. Huang, B., Wu, Y., Li, C., Tang, Q., & Zhang, Y. (2023). Molecular basis and mechanism of action of Albizia julibrissin in depression treatment and clinical application of its formulae. Chinese Herbal Medicines, 15(2), 201–213.
[76]. Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. The World Allergy Organization journal, 5(1), 9–19.
[77]. Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxidative medicine and cellular longevity, 2017, 8416763.
[78]. Czarny, P., Wigner, P., Galecki, P., & Sliwinski, T. (2018). The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Progress in neuro-psychopharmacology & biological psychiatry, 80(Pt C), 309–321.
[79]. Bajpai, A., Verma, A. K., Srivastava, M., & Srivastava, R. (2014). Oxidative stress and major depression. Journal of clinical and diagnostic research: JCDR, 8(12), CC04.
[80]. Bhatt, S., Nagappa, A. N., & Patil, C. R. (2020). Role of oxidative stress in depression. Drug discovery today, 25(7), 1270–1276.
[81]. Bakunina, N., Pariante, C. M., & Zunszain, P. A. (2015). Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology, 144(3), 365–373.
[82]. Trifunovic, S., Stevanovic, I., Milosevic, A., Ristic, N., Janjic, M., Bjelobaba, I., Savic, D., Bozic, I., Jakovljevic, M., Tesovic, K., Laketa, D., & Lavrnja, I. (2021). The Function of the Hypothalamic-Pituitary-Adrenal Axis During Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Frontiers in neuroscience, 15, 649485.
[83]. Rudyk, O., & Aaronson, P. I. (2021). Redox Regulation, Oxidative Stress, and Inflammation in Group 3 Pulmonary Hypertension. Advances in experimental medicine and biology, 1303, 209–241.
[84]. Ryu, D., Jee, H. J., Kim, S. Y., Hwang, S. H., Pil, G. B., & Jung, Y. S. (2022). Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients, 14(16), 3314.
[85]. Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G., & Migliaccio, A. (2020). ROS in cancer therapy: the bright side of the moon. Experimental & molecular medicine, 52(2), 192–203.
[86]. Lee, B., Sur, B., Park, J., Kim, S. H., Kwon, S., Yeom, M., Shim, I., Lee, H., & Hahm, D. H. (2013). Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomolecules & therapeutics, 21(5), 381–390.
[87]. Bansal, Y., Singh, R., Saroj, P., Sodhi, R. K., & Kuhad, A. (2018). Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicology and applied pharmacology, 355, 257–268.
[88]. Li, Y. J., Li, Y. J., Yang, L. D., Zhang, K., Zheng, K. Y., Wei, X. M., Yang, Q., Niu, W. M., Zhao, M. G., & Wu, Y. M. (2018). Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behavioural brain research, 348, 184–191.
[89]. Lu, C. P., Huang, C. Y., Wang, S. H., Chiu, C. H., Li, L. H., Hua, K. F., & Wu, T. H. (2018). Improvement of hyperglycemia in a murine model of insulin resistance and high glucose- and inflammasome-mediated IL-1β expressions in macrophages by silymarin. Chemico-biological interactions, 290, 12–18.
[90]. Ren, L., Tao, W., Zhang, H., Xue, W., Tang, J., Wu, R., Xia, B., Wu, H., & Chen, G. (2016). Two standardized fractions of Gardenia jasminoides Ellis with rapid antidepressant effects are differentially associated with BDNF up-regulation in the hippocampus. Journal of ethnopharmacology, 187, 66–73.
[91]. Li, H. Y., Zhao, Y. H., Zeng, M. J., Fang, F., Li, M., Qin, T. T., Ye, L. Y., Li, H. W., Qu, R., & Ma, S. P. (2017). Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology, 234(22), 3385–3394.
[92]. Zhao, Y., Zhang, Y., & Pan, F. (2015). The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Central-European journal of immunology, 40(1), 11–17.
[93]. Allen, J., Romay-Tallon, R., Brymer, K. J., Caruncho, H. J., & Kalynchuk, L. E. (2018). Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Frontiers in neuroscience, 12, 386.
[94]. Bi, C. C., Guo, S. T., Hu, S. J., Chen, J. Q., Ye, M. F., & Liu, Z. (2022). The microbiota-gut-brain axis and its modulation in the therapy of depression: Comparison of efficacy of conventional drugs and traditional Chinese medicine approaches. Pharmacological Research, 183, Article 106372.
[95]. Yu, M., Jia, H. M., Zhang, T., Shang, H., Zhang, H. W., Ma, L. Y., & Zou, Z. M. (2020). Gut microbiota is the key to the antidepressant effect of Chaihu-Shu-Gan-San. Metabolites, 10(2), 63.
[96]. Liu, X., Lv, M., Wang, Y., Qu, P., Li, S., Yu, Z., & Qin, X. (2021). Anti-depressive effects of Xiaoyaosan, Shugan and Jianpi herbal treatments: role on the gut microbiome of CUMS rats. Phytomedicine, 87, 153581.
[97]. Li, Y. H., Zhang, C. H., Qiu, J., Wang, S. E., Hu, S. Y., Huang, X., ... & Cheng, T. L. (2014). Antidepressant-like effects of Chaihu-Shugan-San via SAPK/JNK signal transduction in rat models of depression. Pharmacognosy magazine, 10(39), 271.
[98]. Lv, S., Zhao, Y., Wang, L., Yu, Y., Li, J., Huang, Y., ... & Sun, P. (2022). Antidepressant Active Components of Bupleurum chinense DC‐Paeonia lactiflora Pall Herb Pair: Pharmacological Mechanisms. BioMed Research International, 2022(1), 1024693.
[99]. Cai, S. B., Zhou, H. Y., Ji, X. Y., Zhang, Q. L., Deng, X. Y., Wang, F., ... & Lin, L. B. (2021). Effects of Bupleurum chinense on the diversity of intestinal flora in depressed mice. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica, 46(16), 4222-4229.
[100]. Zhao, X., Wang, C., Zhang, J. F., Liu, L., Liu, A. M., Ma, Q., ... & Xu, Y. (2014). Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABA A receptor. Psychopharmacology, 231, 2171-2187.
[101]. Maharjan, R., Bustamante, L. D., Ghattas, K. N., Ilyas, S., Al-Refai, R., & Khan, S. (2020). Role of Lifestyle in Neuroplasticity and Neurogenesis in an Aging Brain. Cureus.
[102]. Marzola, P., Melzer, T., Pavesi, E., Gil-Mohapel, J., & Brocardo, P. S. (2023). Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sciences, 13(12), 1610–1610.
[103]. Abbott, L. F., & Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nature neuroscience, 3(11), 1178-1183.
[104]. Leuner, B., & Gould, E. (2010). Structural Plasticity and Hippocampal Function. Annual Review of Psychology, 61(1), 111–140.
[105]. McQueen, K. (2023). Neuroplasticity | Definition, Characteristics & Examples - Lesson | Study.com. Study.com.
[106]. Anand, K. S., & Dhikav, V. (2012). Hippocampus in health and disease: An overview. Annals of Indian Academy of Neurology, 15(4), 239–239.
[107]. Askenasy, J., & Lehmann, J. (2013). Consciousness, brain, neuroplasticity. Frontiers in Psychology, 4, 412.
[108]. Fuster, J. M. (2015). Anatomy of the Prefrontal Cortex. Elsevier EBooks, 9–62.
[109]. Sheline, Y. I., Wang, P. W., Gado, M. H., Csernansky, J. G., & Vannier, M. W. (1996). Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences, 93(9), 3908–3913.
[110]. Bremner, J. D., Narayan, M., Anderson, E. R., Staib, L. H., Miller, H. L., & Charney, D. S. (2000). Hippocampal Volume Reduction in Major Depression | American Journal of Psychiatry. American Journal of Psychiatry, 157(1).
[111]. Sahay, A., Drew, M. R., & Hen, R. (2007). Dentate gyrus neurogenesis and depression. Progress in brain research, 163, 697-822.
[112]. McEwen, B. S., Eiland, L., Hunter, R. G., & Miller, M. M. (2012). Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62(1), 3–12.
[113]. Wang, W., Guo, H., Zhang, S.-X., Li, J., Cheng, K., Bai, S.-J., Yang, D.-Y., Wang, H.-Y., Liang, Z.-H., Liao, L., Sun, L., & Xie, P. (2016). Targeted Metabolomic Pathway Analysis and Validation Revealed Glutamatergic Disorder in the Prefrontal Cortex among the Chronic Social Defeat Stress Mice Model of Depression. Journal of Proteome Research, 15(10), 3784–3792.
[114]. Zhang, E., Suk Yu Yau, Lau, M., Ma, H., Lee, Raymond Chuen-Chung Chang, & Kwok Fai So. (2012). Synaptic Plasticity, But not Hippocampal Neurogenesis, Mediated the Counteractive Effect of Wolfberry on Depression in Rats. Cell Transplantation, 21(12), 2635–2649.
[115]. Yi, L.-T., Li, J., Li, H.-C., Zhou, Y., Su, B.-F., Yang, K.-F., Jiang, M., & Zhang, Y.-T. (2012). Ethanol extracts from Hemerocallis citrina attenuate the decreases of brain-derived neurotrophic factor, TrkB levels in rat induced by corticosterone administration. Journal of Ethnopharmacology, 144(2), 328–334.
[116]. Ding, S., Liu, Q., & Shang, Y. (2022). The Effects and Regulatory Mechanism of Flavonoids from Stems and Leaves of Scutellaria baicalensis Georgi in Promoting Neurogenesis and Improving Memory Impairment Mediated by the BDNF-ERK-CREB Signaling Pathway in Rats. CNS & Neurological Disorders - Drug Targets, 21(4), 354–366.
[117]. Zheng, Q., Yue, P. F., Wu, B., Hu, P. Y., Wu, Z. F., & Yang, M. (2011). Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J Ethnopharmacol, 137(1), 221-225.
[118]. Zheng, M., Zhou, H., Wan, H., Chen, Y. L., & He, Y. (2015). Effects of herbal drugs in Mahuang decoction and their main components on intestinal transport characteristics of Ephedra alkaloids evaluated by a Caco-2 cell monolayer model. J Ethnopharmacol, 164, 22-29.
[119]. Jia, M., Zhou, L., Lou, Y., Yang, X., Zhao, H., Ouyang, X., & Huang, Y. (2023). An analysis of the nutritional effects of Schisandra chinensis components based on mass spectrometry technology. Frontiers in Nutrition, 10, 1227027.
[120]. Wu, Y., Zhao, Z., Yang, Y., Yang, X., Jang, E. Y., Schilaty, N. D., Hedges, D. M., Kim, S. C., Cho, I. J., & Zhao, R. (2014). Effects of the aqueous extract of Schizandra chinensis fruit on ethanol withdrawal-induced anxiety in rats. Chin Med J (Engl), 127(10), 1935-1940.
[121]. Zhang, M., Xu, L., & Yang, H. (2018). Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases. International Journal of Molecular Sciences, 19(7), 1970.
[122]. Sun, Y., Yan, T., Gong, G., Li, Y., Zhang, J., Wu, B., Bi, K., & Jia, Y. (2020). Antidepressant-like effects of Schisandrin on lipopolysaccharide-induced mice : Gut microbiota, short chain fatty acid and TLR4/NF-κB signaling pathway. International Immunopharmacology, 89, 107029.
[123]. Enogieru, A. B., Haylett, W., Hiss, D. C., Bardien, S., & Ekpo, O. E. (2018). Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid Med Cell Longev, 2018, 6241017.
[124]. Sachan, A. K., Vishnoi, G., & Kumar, R. (2016). Need of standardization of herbal medicines in modern era. International Journal of Phytomedicine, 8(3), 300-307.
[125]. Kunle, O. F., Egharevba, H. O., & Ahmadu, P. O. (2012). Standardization of herbal medicines - A review. International Journal of Biodiversity and Conservation, 4(3).
[126]. Scheidt, M. von, Zhao, Y., Kurt, Z., Pan, C., Zeng, L., Yang, X., Heribert Schunkert, & Lusis, A. J. (2017). Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metabolism, 25(2), 248–261.
[127]. Aysha Karim Kiani, Pheby, D., Henehan, G., Brown, R., Sieving, P., Sykora, P., Marks, R., Benedetto Falsini, Natale Capodicasa, Stanislav Miertus, Lorusso, L., Dondossola, D., Gianluca Martino Tartaglia, Mahmut Cerkez Ergoren, Munis Dundar, Michelini, S., Malacarne, D., Bonetti, G., Astrit Dautaj, & Donato, K. (2022). Ethical considerations regarding animal experimentation. HAL (Le Centre Pour La Communication Scientifique Directe), 63(2 Suppl 3), E255–E266.
[128]. Justice, M. J., & Dhillon, P. (2016). Using the mouse to model human disease: increasing validity and reproducibility. Disease Models & Mechanisms, 9(2), 101–103.
[129]. Seok, J., H. Shaw Warren, Cuenca, A. G., Mindrinos, M. N., Baker, H. V., Xu, W., Richards, D. R., McDonald-Smith, G. P., Gao, H., Hennessy, L., Finnerty, C. C., López, C. M., Honari, S., Moore, E. E., Minei, J. P., Cuschieri, J., Bankey, P. E., Johnson, J. L., Sperry, J., & Nathens, A. B. (2013). Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences, 110(9), 3507–3512.
[130]. McCance, D. (2012). Critical animal studies: An introduction. State University of New York Press.
Cite this article
Qin,S.;Hong,J.;Hsu,C.;Kiram,S. (2025). Different Mechanisms of Antidepressant Effect in the Scope of Plant-Based Medicines. Theoretical and Natural Science,115,10-25.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Nabavi, S. M., Daglia, M., Braidy, N., & Nabavi, S. F. (2017). Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutritional neuroscience, 20(3), 180–194.
[2]. Stockmeier, C. A., & Rajkowska, G. (2004). Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues in clinical neuroscience, 6(2), 185–197.
[3]. Kvam, A. K., & Waage, A. (2015). Health-related quality of life in patients with multiple myeloma--does it matter?. Haematologica, 100(6), 704–705.
[4]. Marx, W., Penninx, B. W. J. H., Solmi, M., Furukawa, T. A., Firth, J., Carvalho, A. F., & Berk, M. (2023). Major depressive disorder. Nature reviews. Disease primers, 9(1), 44.
[5]. Elderon, L., & Whooley, M. A. (2013). Depression and cardiovascular disease. Progress in cardiovascular diseases, 55(6), 511–523.
[6]. Zhou, Q. G., Zhu, X. H., Nemes, A. D., & Zhu, D. Y. (2018). Neuronal nitric oxide synthase and affective disorders. IBRO reports, 5, 116–132.
[7]. Scheepens, D. S., van Waarde, J. A., Ten Doesschate, F., Westra, M., Kroes, M. C. W., Schene, A. H., Bockting, C. L. H., Schoevers, R. A., Denys, D. A. J. P., Ruhé, H. G., & van Wingen, G. A. (2020). Effectiveness of Emotional Memory Reactivation vs Control Memory Reactivation Before Electroconvulsive Therapy in Adult Patients With Depressive Disorder: A Randomized Clinical Trial. JAMA network open, 3(8), e2012389.
[8]. Charney D. S. (1998). Monoamine dysfunction and the pathophysiology and treatment of depression. The Journal of clinical psychiatry, 59 Suppl 14, 11–14.
[9]. Pariante, C. M., & Lightman, S. L. (2008). The HPA axis in major depression: classical theories and new developments. Trends in neurosciences, 31(9), 464–468.
[10]. Zunszain, P. A., Anacker, C., Cattaneo, A., Carvalho, L. A., & Pariante, C. M. (2011). Glucocorticoids, cytokines and brain abnormalities in depression. Progress in neuro-psychopharmacology & biological psychiatry, 35(3), 722–729.
[11]. Molendijk, M. L., Spinhoven, P., Polak, M., Bus, B. A., Penninx, B. W., & Elzinga, B. M. (2014). Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Molecular psychiatry, 19(7), 791–800.
[12]. Duman, R. S., Aghajanian, G. K., Sanacora, G., & Krystal, J. H. (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature medicine, 22(3), 238–249.
[13]. Chen, H., Lu, M., Lyu, Q., Shi, L., Zhou, C., Li, M., ... & Ren, L. (2024). Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomedicine & Pharmacotherapy, 175, 116656.
[14]. Ana, R. D., Gliszczyńska, A., Sanchez-Lopez, E., Garcia, M. L., Krambeck, K., Kovacevic, A., & Souto, E. B. (2023). Precision Medicines for Retinal Lipid Metabolism-Related Pathologies. Journal of personalized medicine, 13(4), 635.
[15]. Hui, Y., Vestergaard, G., Deng, L., Kot, W. P., Thymann, T., Brunse, A., & Nielsen, D. S. (2022). Donor-dependent fecal microbiota transplantation efficacy against necrotizing enterocolitis in preterm pigs. NPJ biofilms and microbiomes, 8(1), 48.
[16]. Gong, X., Chang, R., Zou, J., Tan, S., & Huang, Z. (2022). The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Reviews in the neurosciences, 34(3), 313–324.
[17]. Warren, J. B. (2020). The trouble with antidepressants: why the evidence overplays benefits and underplays risks—an essay by John B Warren. bmj, 370.
[18]. Taylor, C., Fricker, A. D., Devi, L. A., & Gomes, I. (2005). Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cellular Signalling, 17(5), 549–557.
[19]. Delgado, P. (2024). Depression: the case for a monoamine deficiency. The Journal of Clinical Psychiatry, 61 Suppl 6.
[20]. Boseley, S. (2018). The drugs do work: antidepressants are effective, study shows. The Guardian, 21, 2018.
[21]. Al-Harbi, K. S. (2012). Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Preference and Adherence, 369–369.
[22]. Westenberg, H. G. M., & Sandner, C. (2006). Tolerability and safety of fluvoxamine and other antidepressants. International Journal of Clinical Practice, 60(4), 482–491.
[23]. Peng, S., Zhou, Y., Lu, M., & Wang, Q. (2022). Review of herbal medicines for the treatment of depression. Natural Product Communications, 17(11), 1934578X221139082.
[24]. van Wyk, A. S., & Prinsloo, G. (2020). Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. South African Journal of Botany, 133, 54-62.
[25]. Briskin, D. P. (2000). Medicinal Plants and Phytomedicines. Linking Plant Biochemistry and Physiology to Human Health. Plant Physiology, 124(2), 507-514.
[26]. Khan, H. (2014). Medicinal plants in light of history: recognized therapeutic modality. Journal of evidence-based complementary & alternative medicine, 19(3), 216-219.
[27]. Patwardhan, B., Vaidya, A.D., Chorghade, M.S., & Joshi, S.P. (2008). Reverse Pharmacology and Systems Approaches for Drug Discovery and Development. Current Bioactive Compounds, 4, 201-212.
[28]. Hirshler, Y., & Doron, R. (2017). Neuroplasticity-related mechanisms underlying the antidepressant-like effects of traditional herbal medicines. Eur Neuropsychopharmacol, 27(10), 945-958.
[29]. Colucci-D'Amato, L., Speranza, L., & Volpicelli, F. (2020). Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. International Journal of Molecular Sciences, 21(20), Article 7777.
[30]. Fries, G. R., Saldana, V. A., Finnstein, J., & Rein, T. (2023). Molecular pathways of major depressive disorder converge on the synapse. Molecular Psychiatry, 28(1), 284-297.
[31]. Najmi, A., Javed, S. A., Al Bratty, M., & Alhazmi, H. A. (2022). Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules, 27(2), 349.
[32]. Hasler, G. (2010). Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry, 9(3), 155-161.
[33]. Hamon, M., & Blier, P. (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry, 45, 54-63.
[34]. Edmondson, D. E. (2014). Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: Biological implications [Article]. Current Pharmaceutical Design, 20(2), 155-160.
[35]. Gonda, X., Dome, P., Neill, J. C., & Tarazi, F. I. (2023). Novel antidepressant drugs: Beyond monoamine targets. CNS Spectr, 28(1), 6-15.
[36]. Leonard, B., & Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression [Review]. Neuroscience and Biobehavioral Reviews, 36(2), 764-785.
[37]. Maes, M., Kubera, M., Obuchowiczwa, E., Goehler, L., & Brzeszcz, J. (2011). Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways [Review]. Neuroendocrinology Letters, 32(1), 7-24.
[38]. Jiang, Y., Zou, D., Li, Y. M., Gu, S. M., Dong, J., Ma, X. J., Xu, S. J., Wang, F. S., & Huang, J. H. (2022). Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals, 15(10), Article 1203.
[39]. Li, J. (2007). Flora of China. Harvard Papers in Botany, 13(2), 301-302, 302.
[40]. Sloley, B. D., Urichuk, L. J., Morley, P., Durkin, J., Shan, J. J., Pang, P. K., & Coutts, R. T. (2000). Identification of kaempferol as a monoamine oxidase inhibitor and potential Neuroprotectant in extracts of Ginkgo biloba leaves. J Pharm Pharmacol, 52(4), 451-459.
[41]. Gidaro, M. C., Astorino, C., Petzer, A., Carradori, S., Alcaro, F., Costa, G., Artese, A., Rafele, G., Russo, F. M., Petzer, J. P., & Alcaro, S. (2016). Kaempferol as Selective Human MAO-A Inhibitor: Analytical Detection in Calabrian Red Wines, Biological and Molecular Modeling Studies. J Agric Food Chem, 64(6), 1394-1400.
[42]. Lin, H.-Y., Tsai, J.-C., Wu, L.-Y., & Peng, W.-H. (2020). Reveals of New Candidate Active Components in Hemerocallis Radix and Its Anti-Depression Action of Mechanism Based on Network Pharmacology Approach. International Journal of Molecular Sciences, 21(5), 1868.
[43]. Yan, T., Nian, T., Liao, Z., Xiao, F., Wu, B., Bi, K., He, B., & Jia, Y. (2020). Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int J Biol Macromol, 144, 427-440.
[44]. Shen, F., Song, Z., Xie, P., Li, L., Wang, B., Peng, D., & Zhu, G. (2021). Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. Journal of ethnopharmacology, 275, 114164.
[45]. Vreeburg, S. A., Hoogendijk, W. J., van Pelt, J., Derijk, R. H., Verhagen, J. C., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of general psychiatry, 66(6), 617–626.
[46]. Ancelin, M. L., Scali, J., Norton, J., Ritchie, K., Dupuy, A. M., Chaudieu, I., & Ryan, J. (2017). Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology, 77, 90–94.
[47]. Pan, Y., Kong, L. D., Li, Y. C., Xia, X., Kung, H. F., & Jiang, F. X. (2007). Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacology, biochemistry, and behavior, 87(1), 130–140.
[48]. Po, K. K., Leung, J. W., Chan, J. N., Fung, T. K., Sánchez-Vidaña, D. I., Sin, E. L., So, K. F., Lau, B. W., & Siu, A. M. (2017). Protective effect of Lycium Barbarum polysaccharides on dextromethorphan-induced mood impairment and neurogenesis suppression. Brain research bulletin, 134, 10–17.
[49]. Kim, D. H., Moon, Y. S., Jung, J. S., Min, S. K., Son, B. K., Suh, H. W., & Song, D. K. (2003). Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neuroscience letters, 343(1), 62–66.
[50]. Chen, Y., Han, T., Rui, Y., Yin, M., Qin, L., & Zheng, H. (2005). Effects of total triterpenes of Centella asiatica on the corticosterone levels in serum and contents of monoamine in depression rat brain. Zhong yao cai= Zhongyaocai= Journal of Chinese medicinal materials, 28(6), 492-496.
[51]. Hu, Y., Liao, H. B., Liu, P., Guo, D. H., & Wang, Y. Y. (2009). Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine, 7(7), 667–670.
[52]. Hayley, S., Hakim, A. M., & Albert, P. R. (2021). Depression, dementia and immune dysregulation. Brain, 144(3), 746-760.
[53]. Milaneschi, Y., Kappelmann, N., Ye, Z., Lamers, F., Moser, S., Jones, P. B., ... & Khandaker, G. M. (2021). Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Molecular psychiatry, 26(12), 7393-7402.
[54]. Liu, B., Xu, C., Wu, X., Liu, F., Du, Y., Sun, J., ... & Dong, J. (2015). Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience, 294, 193-205.
[55]. Li, H., Lin, S., Qin, T., Li, H., Ma, Z., & Ma, S. (2017). Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-κB regulating NLRP3 signal pathway. International immunopharmacology, 53, 24-32.
[56]. Singh, J. C. H., Kakalij, R. M., Kshirsagar, R. P., Kumar, B. H., Komakula, S. S. B., & Diwan, P. V. (2015). Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharmaceutical biology, 53(5), 630-636.
[57]. Dang, H., Chen, Y., Liu, X., Wang, Q., Wang, L., Jia, W., & Wang, Y. (2009). Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33(8), 1417-1424.
[58]. Kang, A., Hao, H., Zheng, X., Liang, Y., Xie, Y., Xie, T., ... & Wang, G. (2011). Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. Journal of neuroinflammation, 8, 1-14.
[59]. Beurel, E., Toups, M., & Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron, 107(2), 234-256.
[60]. Ramaswamy, S., & Kordower, J. H. (2008). Gene and Cellular Transplantation Therapies for Huntington's Disease. In CNS Regeneration (pp. 267-294). Academic Press.
[61]. Bathina, S., & Das, U. N. (2015). Brain-derived neurotrophic factor and its clinical implications. Archives of Medical Science, 6, 1164–1178.
[62]. Azman, K. F., & Zakaria, R. (2022). Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. International Journal of Molecular Sciences, 23(12), 6827–6827.
[63]. Lambert, W. M., Xu, C.-F., Neubert, T., Chao, M., Garabedian, M., & Jeanneteau, F. (2013). Brain-Derived Neurotrophic Factor Signaling Rewrites the Glucocorticoid Transcriptome via Glucocorticoid Receptor Phosphorylation. Molecular and Cellular Biology, 33(18), 3700–3714.
[64]. Correia, A. S., Cardoso, A., & Vale, N. (2023). BDNF Unveiled: Exploring Its Role in Major Depression Disorder Serotonergic Imbalance and Associated Stress Conditions. Pharmaceutics, 15(8), 2081–2081.
[65]. Sangiovanni, E., Brivio, P., Dell’Agli, M., & Calabrese, F. (2017). Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plasticity, 2017, 1–19.
[66]. Brunoni, A. R., Lopes, M., & Fregni, F. (2008). A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology, 11(8), 1169–1180.
[67]. Liu, X., Li, P., Ma, X., Zhang, J., Sun, X., Luo, X., & Zhang, Y. (2022). Association between plasma levels of BDNF and GDNF and the diagnosis, treatment response in first-episode MDD. Journal of Affective Disorders, 315, 190–197.
[68]. Hsu, Y.-W., Tsai, C.-F., Chen, W.-K., Ho, Y.-C., & Lu, F.-J. (2011). Determination of lutein and zeaxanthin and antioxidant capacity of supercritical carbon dioxide extract from daylily (Hemerocallis disticha). Food Chemistry, 129(4), 1813–1818.
[69]. Liu, X.-L., Luo, L., Liu, B.-B., Li, J., Geng, D., Liu, Q., & Yi, L.-T. (2014). Ethanol extracts from Hemerocallis citrina attenuate the upregulation of proinflammatory cytokines and indoleamine 2,3-dioxygenase in rats. Journal of Ethnopharmacology, 153(2), 484–490.
[70]. Fotsing, D., Ngoupaye, G. T., Ouafo, A. C., Stephanie, Kenneth, Y. A., & Elisabeth Ngo Bum. (2017). Effects of Gladiolus dalenii on the Stress-Induced Behavioral, Neurochemical, and Reproductive Changes in Rats. Frontiers in Pharmacology, 8.
[71]. Ngoupaye, G. T., Bum, E. N., & Mark, W. (2013). Antidepressant-like effects of the aqueous macerate of the bulb of Gladiolus dalenii Van Geel (Iridaceae) in a rat model of epilepsy-associated depression. BMC Complementary and Alternative Medicine, 13(1).
[72]. Yi, L., Zhang, M., Cheng, J., Wan, H., Li, C., Zhu, J., Zhang, Q., Liu, Q., & Xu, G. (2021). Antidepressant‐like Effects of Degraded Porphyran Isolated from Porphyra haitanensis. Molecular Nutrition & Food Research, 65(9).
[73]. Lee, H. W., Ryu, H. W., Kang, M.-G., Park, D., Lee, H., Shin, H. M., Oh, S.-R., & Kim, H. (2017). Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. International Journal of Biological Macromolecules, 97, 598–605.
[74]. Yu, H., Yin, Z., Yang, S., Ma, S., & Qu, R. (2015). Baicalin Reverses Depressive‐Like Behaviours and Regulates Apoptotic Signalling Induced by Olfactory Bulbectomy. Phytotherapy Research, 30(3), 469–475.
[75]. Huang, B., Wu, Y., Li, C., Tang, Q., & Zhang, Y. (2023). Molecular basis and mechanism of action of Albizia julibrissin in depression treatment and clinical application of its formulae. Chinese Herbal Medicines, 15(2), 201–213.
[76]. Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., & Kalayci, O. (2012). Oxidative stress and antioxidant defense. The World Allergy Organization journal, 5(1), 9–19.
[77]. Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxidative medicine and cellular longevity, 2017, 8416763.
[78]. Czarny, P., Wigner, P., Galecki, P., & Sliwinski, T. (2018). The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Progress in neuro-psychopharmacology & biological psychiatry, 80(Pt C), 309–321.
[79]. Bajpai, A., Verma, A. K., Srivastava, M., & Srivastava, R. (2014). Oxidative stress and major depression. Journal of clinical and diagnostic research: JCDR, 8(12), CC04.
[80]. Bhatt, S., Nagappa, A. N., & Patil, C. R. (2020). Role of oxidative stress in depression. Drug discovery today, 25(7), 1270–1276.
[81]. Bakunina, N., Pariante, C. M., & Zunszain, P. A. (2015). Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology, 144(3), 365–373.
[82]. Trifunovic, S., Stevanovic, I., Milosevic, A., Ristic, N., Janjic, M., Bjelobaba, I., Savic, D., Bozic, I., Jakovljevic, M., Tesovic, K., Laketa, D., & Lavrnja, I. (2021). The Function of the Hypothalamic-Pituitary-Adrenal Axis During Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Frontiers in neuroscience, 15, 649485.
[83]. Rudyk, O., & Aaronson, P. I. (2021). Redox Regulation, Oxidative Stress, and Inflammation in Group 3 Pulmonary Hypertension. Advances in experimental medicine and biology, 1303, 209–241.
[84]. Ryu, D., Jee, H. J., Kim, S. Y., Hwang, S. H., Pil, G. B., & Jung, Y. S. (2022). Luteolin-7-O-Glucuronide Improves Depression-like and Stress Coping Behaviors in Sleep Deprivation Stress Model by Activation of the BDNF Signaling. Nutrients, 14(16), 3314.
[85]. Perillo, B., Di Donato, M., Pezone, A., Di Zazzo, E., Giovannelli, P., Galasso, G., Castoria, G., & Migliaccio, A. (2020). ROS in cancer therapy: the bright side of the moon. Experimental & molecular medicine, 52(2), 192–203.
[86]. Lee, B., Sur, B., Park, J., Kim, S. H., Kwon, S., Yeom, M., Shim, I., Lee, H., & Hahm, D. H. (2013). Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomolecules & therapeutics, 21(5), 381–390.
[87]. Bansal, Y., Singh, R., Saroj, P., Sodhi, R. K., & Kuhad, A. (2018). Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicology and applied pharmacology, 355, 257–268.
[88]. Li, Y. J., Li, Y. J., Yang, L. D., Zhang, K., Zheng, K. Y., Wei, X. M., Yang, Q., Niu, W. M., Zhao, M. G., & Wu, Y. M. (2018). Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behavioural brain research, 348, 184–191.
[89]. Lu, C. P., Huang, C. Y., Wang, S. H., Chiu, C. H., Li, L. H., Hua, K. F., & Wu, T. H. (2018). Improvement of hyperglycemia in a murine model of insulin resistance and high glucose- and inflammasome-mediated IL-1β expressions in macrophages by silymarin. Chemico-biological interactions, 290, 12–18.
[90]. Ren, L., Tao, W., Zhang, H., Xue, W., Tang, J., Wu, R., Xia, B., Wu, H., & Chen, G. (2016). Two standardized fractions of Gardenia jasminoides Ellis with rapid antidepressant effects are differentially associated with BDNF up-regulation in the hippocampus. Journal of ethnopharmacology, 187, 66–73.
[91]. Li, H. Y., Zhao, Y. H., Zeng, M. J., Fang, F., Li, M., Qin, T. T., Ye, L. Y., Li, H. W., Qu, R., & Ma, S. P. (2017). Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology, 234(22), 3385–3394.
[92]. Zhao, Y., Zhang, Y., & Pan, F. (2015). The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Central-European journal of immunology, 40(1), 11–17.
[93]. Allen, J., Romay-Tallon, R., Brymer, K. J., Caruncho, H. J., & Kalynchuk, L. E. (2018). Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Frontiers in neuroscience, 12, 386.
[94]. Bi, C. C., Guo, S. T., Hu, S. J., Chen, J. Q., Ye, M. F., & Liu, Z. (2022). The microbiota-gut-brain axis and its modulation in the therapy of depression: Comparison of efficacy of conventional drugs and traditional Chinese medicine approaches. Pharmacological Research, 183, Article 106372.
[95]. Yu, M., Jia, H. M., Zhang, T., Shang, H., Zhang, H. W., Ma, L. Y., & Zou, Z. M. (2020). Gut microbiota is the key to the antidepressant effect of Chaihu-Shu-Gan-San. Metabolites, 10(2), 63.
[96]. Liu, X., Lv, M., Wang, Y., Qu, P., Li, S., Yu, Z., & Qin, X. (2021). Anti-depressive effects of Xiaoyaosan, Shugan and Jianpi herbal treatments: role on the gut microbiome of CUMS rats. Phytomedicine, 87, 153581.
[97]. Li, Y. H., Zhang, C. H., Qiu, J., Wang, S. E., Hu, S. Y., Huang, X., ... & Cheng, T. L. (2014). Antidepressant-like effects of Chaihu-Shugan-San via SAPK/JNK signal transduction in rat models of depression. Pharmacognosy magazine, 10(39), 271.
[98]. Lv, S., Zhao, Y., Wang, L., Yu, Y., Li, J., Huang, Y., ... & Sun, P. (2022). Antidepressant Active Components of Bupleurum chinense DC‐Paeonia lactiflora Pall Herb Pair: Pharmacological Mechanisms. BioMed Research International, 2022(1), 1024693.
[99]. Cai, S. B., Zhou, H. Y., Ji, X. Y., Zhang, Q. L., Deng, X. Y., Wang, F., ... & Lin, L. B. (2021). Effects of Bupleurum chinense on the diversity of intestinal flora in depressed mice. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica, 46(16), 4222-4229.
[100]. Zhao, X., Wang, C., Zhang, J. F., Liu, L., Liu, A. M., Ma, Q., ... & Xu, Y. (2014). Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABA A receptor. Psychopharmacology, 231, 2171-2187.
[101]. Maharjan, R., Bustamante, L. D., Ghattas, K. N., Ilyas, S., Al-Refai, R., & Khan, S. (2020). Role of Lifestyle in Neuroplasticity and Neurogenesis in an Aging Brain. Cureus.
[102]. Marzola, P., Melzer, T., Pavesi, E., Gil-Mohapel, J., & Brocardo, P. S. (2023). Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sciences, 13(12), 1610–1610.
[103]. Abbott, L. F., & Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nature neuroscience, 3(11), 1178-1183.
[104]. Leuner, B., & Gould, E. (2010). Structural Plasticity and Hippocampal Function. Annual Review of Psychology, 61(1), 111–140.
[105]. McQueen, K. (2023). Neuroplasticity | Definition, Characteristics & Examples - Lesson | Study.com. Study.com.
[106]. Anand, K. S., & Dhikav, V. (2012). Hippocampus in health and disease: An overview. Annals of Indian Academy of Neurology, 15(4), 239–239.
[107]. Askenasy, J., & Lehmann, J. (2013). Consciousness, brain, neuroplasticity. Frontiers in Psychology, 4, 412.
[108]. Fuster, J. M. (2015). Anatomy of the Prefrontal Cortex. Elsevier EBooks, 9–62.
[109]. Sheline, Y. I., Wang, P. W., Gado, M. H., Csernansky, J. G., & Vannier, M. W. (1996). Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences, 93(9), 3908–3913.
[110]. Bremner, J. D., Narayan, M., Anderson, E. R., Staib, L. H., Miller, H. L., & Charney, D. S. (2000). Hippocampal Volume Reduction in Major Depression | American Journal of Psychiatry. American Journal of Psychiatry, 157(1).
[111]. Sahay, A., Drew, M. R., & Hen, R. (2007). Dentate gyrus neurogenesis and depression. Progress in brain research, 163, 697-822.
[112]. McEwen, B. S., Eiland, L., Hunter, R. G., & Miller, M. M. (2012). Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62(1), 3–12.
[113]. Wang, W., Guo, H., Zhang, S.-X., Li, J., Cheng, K., Bai, S.-J., Yang, D.-Y., Wang, H.-Y., Liang, Z.-H., Liao, L., Sun, L., & Xie, P. (2016). Targeted Metabolomic Pathway Analysis and Validation Revealed Glutamatergic Disorder in the Prefrontal Cortex among the Chronic Social Defeat Stress Mice Model of Depression. Journal of Proteome Research, 15(10), 3784–3792.
[114]. Zhang, E., Suk Yu Yau, Lau, M., Ma, H., Lee, Raymond Chuen-Chung Chang, & Kwok Fai So. (2012). Synaptic Plasticity, But not Hippocampal Neurogenesis, Mediated the Counteractive Effect of Wolfberry on Depression in Rats. Cell Transplantation, 21(12), 2635–2649.
[115]. Yi, L.-T., Li, J., Li, H.-C., Zhou, Y., Su, B.-F., Yang, K.-F., Jiang, M., & Zhang, Y.-T. (2012). Ethanol extracts from Hemerocallis citrina attenuate the decreases of brain-derived neurotrophic factor, TrkB levels in rat induced by corticosterone administration. Journal of Ethnopharmacology, 144(2), 328–334.
[116]. Ding, S., Liu, Q., & Shang, Y. (2022). The Effects and Regulatory Mechanism of Flavonoids from Stems and Leaves of Scutellaria baicalensis Georgi in Promoting Neurogenesis and Improving Memory Impairment Mediated by the BDNF-ERK-CREB Signaling Pathway in Rats. CNS & Neurological Disorders - Drug Targets, 21(4), 354–366.
[117]. Zheng, Q., Yue, P. F., Wu, B., Hu, P. Y., Wu, Z. F., & Yang, M. (2011). Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J Ethnopharmacol, 137(1), 221-225.
[118]. Zheng, M., Zhou, H., Wan, H., Chen, Y. L., & He, Y. (2015). Effects of herbal drugs in Mahuang decoction and their main components on intestinal transport characteristics of Ephedra alkaloids evaluated by a Caco-2 cell monolayer model. J Ethnopharmacol, 164, 22-29.
[119]. Jia, M., Zhou, L., Lou, Y., Yang, X., Zhao, H., Ouyang, X., & Huang, Y. (2023). An analysis of the nutritional effects of Schisandra chinensis components based on mass spectrometry technology. Frontiers in Nutrition, 10, 1227027.
[120]. Wu, Y., Zhao, Z., Yang, Y., Yang, X., Jang, E. Y., Schilaty, N. D., Hedges, D. M., Kim, S. C., Cho, I. J., & Zhao, R. (2014). Effects of the aqueous extract of Schizandra chinensis fruit on ethanol withdrawal-induced anxiety in rats. Chin Med J (Engl), 127(10), 1935-1940.
[121]. Zhang, M., Xu, L., & Yang, H. (2018). Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases. International Journal of Molecular Sciences, 19(7), 1970.
[122]. Sun, Y., Yan, T., Gong, G., Li, Y., Zhang, J., Wu, B., Bi, K., & Jia, Y. (2020). Antidepressant-like effects of Schisandrin on lipopolysaccharide-induced mice : Gut microbiota, short chain fatty acid and TLR4/NF-κB signaling pathway. International Immunopharmacology, 89, 107029.
[123]. Enogieru, A. B., Haylett, W., Hiss, D. C., Bardien, S., & Ekpo, O. E. (2018). Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid Med Cell Longev, 2018, 6241017.
[124]. Sachan, A. K., Vishnoi, G., & Kumar, R. (2016). Need of standardization of herbal medicines in modern era. International Journal of Phytomedicine, 8(3), 300-307.
[125]. Kunle, O. F., Egharevba, H. O., & Ahmadu, P. O. (2012). Standardization of herbal medicines - A review. International Journal of Biodiversity and Conservation, 4(3).
[126]. Scheidt, M. von, Zhao, Y., Kurt, Z., Pan, C., Zeng, L., Yang, X., Heribert Schunkert, & Lusis, A. J. (2017). Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metabolism, 25(2), 248–261.
[127]. Aysha Karim Kiani, Pheby, D., Henehan, G., Brown, R., Sieving, P., Sykora, P., Marks, R., Benedetto Falsini, Natale Capodicasa, Stanislav Miertus, Lorusso, L., Dondossola, D., Gianluca Martino Tartaglia, Mahmut Cerkez Ergoren, Munis Dundar, Michelini, S., Malacarne, D., Bonetti, G., Astrit Dautaj, & Donato, K. (2022). Ethical considerations regarding animal experimentation. HAL (Le Centre Pour La Communication Scientifique Directe), 63(2 Suppl 3), E255–E266.
[128]. Justice, M. J., & Dhillon, P. (2016). Using the mouse to model human disease: increasing validity and reproducibility. Disease Models & Mechanisms, 9(2), 101–103.
[129]. Seok, J., H. Shaw Warren, Cuenca, A. G., Mindrinos, M. N., Baker, H. V., Xu, W., Richards, D. R., McDonald-Smith, G. P., Gao, H., Hennessy, L., Finnerty, C. C., López, C. M., Honari, S., Moore, E. E., Minei, J. P., Cuschieri, J., Bankey, P. E., Johnson, J. L., Sperry, J., & Nathens, A. B. (2013). Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences, 110(9), 3507–3512.
[130]. McCance, D. (2012). Critical animal studies: An introduction. State University of New York Press.









