1. Introduction
Habits are automatic, inflexible, and unconscious responses formed through repeated practice [1-4]. Automation conserves cognitive resources, reduces deliberation, and enhances task efficiency. However, their inflexibility can lead to maladaptive outcomes, potentially evolving into compulsive or addictive behaviors under extreme reinforcement [2,3]. The rigidity of habitual behaviors is commonly observed in conditions such as addiction and obsessive-compulsive disorder (OCD), and behavioral inflexibility reminiscent of habit dysregulation has also been observed in autism spectrum disorders (ASD). Though not confirmed causally, habit-circuit dysfunctions are strongly implicated [2, 4]. Elucidating and modulating the neural mechanisms of habits is essential for treating behavioral inflexibility in clinical conditions and promoting adaptive behavior in healthy populations.
Despite progress, habit research struggles with conceptual clarity and methodological precision in capturing neural dynamics. Current mainstream methodologies predominantly regard neural activities as final outputs, employing event-based labeling as the standard analytical unit [5]. This paradigm neglects behavioral continuity and feedback loops, processes central to the formation and persistence of habits [2,4-6]. Furthermore, although the brain regions involved in habit formation have been repeatedly identified and validated [1,4,6,7], critical questions remain unresolved. The exact neuronal populations participating in microcircuits, the topological organization underlying habit storage, such as spatial patterns within regions or connectivity across circuits, and the precise mechanisms of inter-regional cooperation among different brain structures remain largely elusive [2].
Addressing these questions requires advances in theoretical models and experimental tools that enable multi-regional interventions and recordings, with cellular spatial resolution and millisecond-level temporal precision. Brain-computer interfaces (BCIs), especially closed-loop BCIs (CLBCIs), offer promising solutions by meeting these methodological demands. Recent advances in subcortical monitoring and single-cell precision have empowered BCI technology, particularly closed-loop platforms, to demonstrate substantial potential for transforming habit research [8, 9].
This study presents a comprehensive literature synthesis of recent findings on the neural mechanisms underlying habitual behavior and advances in BCI technologies. By evaluating the capacity of BCIs, particularly CLBCIs, to address longstanding methodological limitations, the review highlights their relevance to both basic neuroscience and clinical applications.
2. Neural mechanisms of habit formation
2.1. The classic DMS–DLS dichotomy
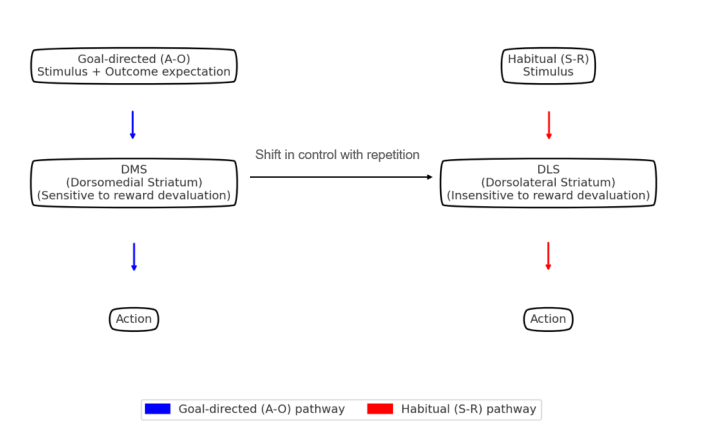
According to the dominant framework illustrated in Fig. 1, habitual behavior is thought to emerge through a progressive shift from goal-directed action-outcome (A-O) processes mediated by the dorsomedial striatum (DMS, i.e., anterior caudate in humans) to stimulus-response (S-R) processes mediated by the dorsolateral striatum (DLS, i.e., posterior lateral putamen in humans). As this shift progresses, repeated performance renders actions increasingly automatic and inflexible [4,10,11,12]. However, growing evidence challenges this strict dichotomy. Neural recordings reveal overlapping task-related activity in both DMS and DLS [4], and dopamine dynamics do not shift uniformly from DMS to DLS with habit formation. In habitual animals, DMS dopamine shows phase-specific modulation, while DLS signals remain largely unchanged (Fig. 2). Optogenetic stimulation of DMS dopamine accelerates habit acquisition [1]. Behavioral studies further demonstrate that goal-directed and habitual processes may alternate within a single trial [4]. Thus, rather than acting as a fixed “habit region,” the DLS appears to operate as a dynamically recruited node within an evolving control network. While DMS inactivation induces habitual-like responses [13] and DLS inactivation can restore goal-directed behavior [10], the strict division of labor between these regions risks oversimplifying the distributed nature of habit control. Instead, DMS and DLS are better conceptualized as interacting modules whose engagement may shift based on task phase or motivational state [2,14].
2.2. Habit as a multi-region dynamic network
Evidence from rodents and primates shows that habits involve a broader cortico-striatal-thalamic-limbic network beyond DLS and DMS, as shown in Fig. 3. Outcome-independent behaviors rely on DLS, the substantia nigra pars compacta (SNc), the infralimbic (IL) cortex, the central nucleus of the amygdala (CeA), and the perifascicular nucleus (PF) of the thalamus. Goal-directed behavior relies on the prelimbic (PL) cortex, orbitofrontal cortex (OFC), and DMS [2, 6].
The PL–DMS pathway mediates A-O sensitivity; its lesion impairs goal-directed behavior and blocks outcome devaluation effects [2,11]. IL activity increases with overtraining and promotes habitual control via projections to DLS. IL modulation inhibits habit formation [15]. OFC-DMS projections encode reward value. Plasticity at OFC-DMS synapses shifts behavior toward habitual responding [2]. OFC modulates the balance between goal-directed and habitual systems [2,7]. Silencing OFC-DMS projections blocks stimulus-reward updating and induces behavioral rigidity [14]. The PF-DMS pathway supports flexibility, and thalamostriatal input sustains adaptive action selection [2]. Though not projecting directly to DLS, the basolateral (BLA) and central (CeA) amygdala influence habits via multisynaptic circuits. BLA is active early and likely modulates DLS via ventral-to-dorsal striatal pathways. CeA dominates later and encodes valence [2]. VTA and SNc modulate habits through dopamine; reward amplifies their striatal influence [2]. Inhibiting the left primary motor cortex (M1) via transcranial magnetic stimulation (TMS) enhances control, suggesting that M1 suppresses habits via corticostriatal regulation [7]. DLS forms a self-reinforcing loop: it inhibits substantia nigra pars reticulata (SNr), disinhibiting PF, which feeds back to DLS to consolidate habits [6].
2.3. Beyond simple S–R encoding
Although convenient, the S-R framework oversimplifies the neural basis of habitual behavior. Direct evidence for S-R coding within the striatum is surprisingly sparse [4]. DLS neurons instead encode motor “chunks”, action sequences bracketed by neural activity peaks at the start and end. This stability across stimuli suggests that habits operate independently of fixed S-R combinations [2,4]. Habits are not entirely goal-free, as DLS neurons retain sensitivity to reward feedback. Some neurons fire during actions, others after reward consumption or task completion. Overtraining, their activity shifts from error-responsiveness to reward selectivity, reinforcing reward-driven behavior [4,18]. This suggests that habitual circuits are not outcome-insensitive but integrate feedback with a bias toward reward-consistent patterns. Thus, habitual circuits appear outcome-biased rather than outcome-blind. They reinforce historically rewarded behaviors, creating a rigidity that mimics S-R automation. This supports the view that goal-directed and habitual behaviors are not categorically distinct but exist along a continuum of control intensity and circuit dominance [16].
![Figure 2. Dopamine dynamics in ventromedial striatum (VMS), DMS, and DLS during reward-seeking behavior [1].](https://file.ewadirect.com/press/media/markdown/document-image2_Ka9xr4o.png)
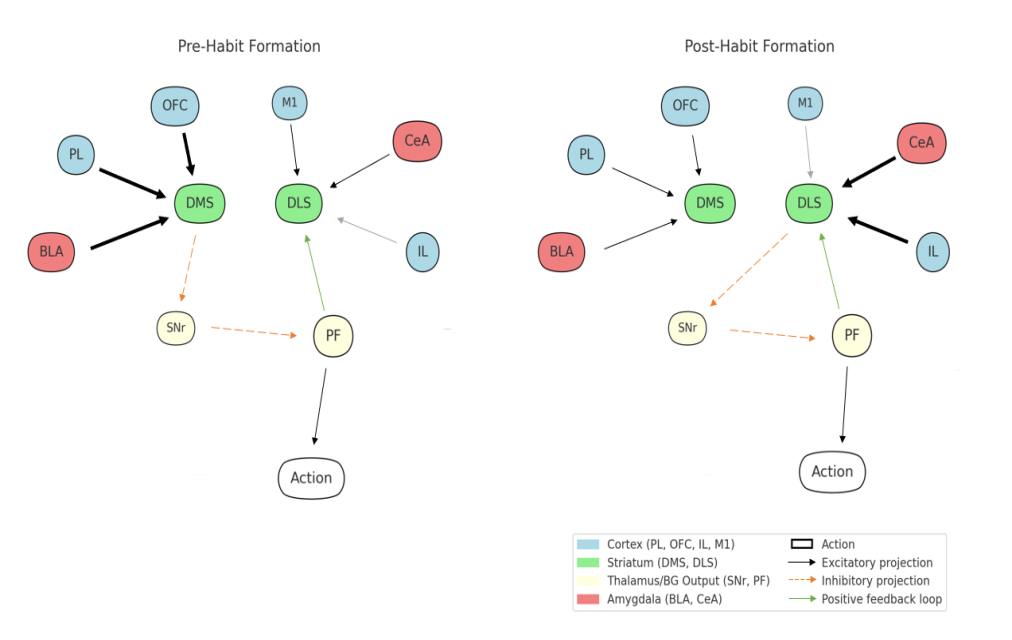
Pre-habit actions are gated by PL, OFC, and BLA projections to DMS, facilitating SNr disinhibition of PF. Post-habit, IL and CeA inputs dominate DLS control, with a strengthened DLS→SNr→PF→DLS loop sustaining habitual execution. Arrow thickness reflects changes in connection strength: M1 →DLS, BLA→DMS, PL→DMS, and OFC→DMS projections weaken, while IL→DLS and CeA→DLS pathways strengthen. The diagram does not differentiate between direct and indirect projections.
3. Limitations of current research paradigms and tools
3.1. Shortcomings of linear models
Most habit studies adopt the S-R framework, assuming behavior results from linear causality, stimuli processed by internal circuits directly elicit responses. This approach either manipulates stimuli or neural activity to observe behavioral changes [2,4,14,15] or records neural changes during S-R updates [2,4], helping to identify key habit-related regions and their activity patterns. However, this linear model is inadequate as habit formation engages multiple interacting regions through parallel circuits [2] and complex positive feedback loops [6]. Reducing internal neural processing to a single unit, without considering dynamic interactions, inevitably leads to fragmented conclusions.
Many updates retain a linear view, enriching only the variables between stimulus and response, or adding cognitive processes [5]. Henry’s hierarchical control model [5] (Fig. 4) offers a deeper alternative. It posits that behavior arises from minimizing deviation from goal states (attractors) via recurrent negative feedback, rather than executing fixed outputs. This structure has been validated in quadrupedal robotic systems (Fig. 5), supporting its biological plausibility [17]. Behavior, then, reflects continuous internal adjustment rather than stimulus-driven execution. Thus, rather than fixed outputs, habits likely reflect reorganization within hierarchical frameworks [4]. Therefore, models must evolve beyond simple linear causality to encompass hierarchical, feedback-driven dynamics.
3.2. Unsolved neural mechanisms
While key brain regions involved in habit formation are identified, the specific neuronal populations underlying distinct habitual behaviors remain unclear. These regions contain diverse projection neurons and interneurons [18]. Although they modulate striatal output across goal-directed and habitual modes, their precise interactions remain unresolved (Fig. 6). SPN activation during behavior is localized rather than global across the striatum [2]. The DLS–SNr–PF–DLS circuit further subdivides into parallel loops, suggesting modular encoding of distinct action sequences [6]. Whether such organizations vary across individuals or permit selective control of specific habits remains unknown.
The mechanisms of interregional influence are poorly defined. Although IL’s role in habit arbitration is validated by optogenetics [15], its lack of direct projections to DLS suggests unidentified intermediaries [18]. Whether habit dominance suppresses cognitive control via reduced prefrontal activity or enhanced DLS/IL drive is also unknown [4]. Integration of multi-regional signals into coherent habitual behavior also remains poorly understood [2,18]. Although within-trial dynamics between cognitive and habitual control have been observed [4], real-time tracking of habitual dominance is limited.
Future studies must identify microcircuits at the single-neuron level, map interregional information integration, and characterize closed-loop feedback dynamics. These objectives demand tools with millisecond precision, cellular resolution, multi-region recording, real-time state tracking, and closed-loop modulation.
![Figure 4. Illustration of a multi-level control hierarchy [5].](https://file.ewadirect.com/press/media/markdown/document-image4_eQCZRr0.png)
Each layer comprises a comparator that adjusts outputs to minimize deviations in controlled variables. Error signals propagate upward, while corrective outputs are generated downward, forming interlinked negative feedback loops. The system maintains behavioral stability through parallel, continuous regulation without relying on discrete S–R mappings.
![Figure 5. Hierarchical control architecture implemented in a quadruped robot [17].](https://file.ewadirect.com/press/media/markdown/document-image5_sNVrhhQ.png)
Each module continuously compares perceived and goal states, adjusting motor outputs to minimize error. Lower-level controllers regulate motor variables, while higher layers coordinate complex behaviors. Negative feedback through the environment allows real-time adaptation across layers.
![Figure 6. Cell-type composition and interactions within DMS and DLS [18].](https://file.ewadirect.com/press/media/markdown/document-image6_1xRvYzM.png)
Both regions contain direct-pathway spiny projection neurons (dSPNs), indirect-pathway spiny projection neurons (iSPNs), fast-spiking interneurons (FSIs), tyrosine hydroxylase-expressing interneurons (THINs), and cholinergic interneurons (CINs). FSIs modulate SPN activity in both regions but exert opposite functional effects. THINs and CINs are required for goal-directed control in DMS, while their roles in DLS remain unclear. Several key interactions (dashed arrows) remain unresolved.
4. The progress and potential of BCI
4.1. Overview of BCI and closed-loop advances
BCIs provide a direct communication pathway between the brain and external devices, enabling decoding of brain states and modulation of neural activity [19]. BCIs have become powerful tools for understanding brain functions [20] and enhancing them [21]. CLBCIs represent a key advancement, allowing dynamic adjustment of outputs based on real-time feedback [22].
Despite their potential, the application of BCIs to habit research remains constrained by several challenges. Current systems are expensive and time-consuming, particularly for closed-loop operations [23]. Traditional methods often struggle to model the spatial topology of brain networks [24], suffer from insufficient spatial resolution to target single neurons [8], and face difficulties in recording from multiple deep brain regions such as the striatum [22]. Moreover, invasive BCIs often encounter signal degradation over time due to gliosis and other tissue responses [22].
Recent advances have begun to address these limitations. Algorithm-hardware co-design now enables precise, low-power closed-loop BCIs [22]. To overcome CNN/RNN limitations in EEG analysis, forward-mechanism fused graph convolutional networks (F-FGCN) better capture spatial relationships across channels [24]. Flexible high-density microelectrode arrays (HDMEAs) provide single-neuron resolution [8], and MEMS-based devices allow chronic, multi-region implantation in primates for real-time dopamine monitoring [25]. Tools like the Neuroscroll probe further support high-resolution, long-term recordings across brain depths [9].
4.2. BCI in habit research and clinical application
BCI-related technologies have shown preliminary utility in habit research. In mice, optogenetic IL manipulation enabled reversible, online modulation of habitual behavior [15]. In humans, inhibitory continuous theta burst stimulation (cTBS) over M1 suppressed habitual responses and enhanced action precision [7]. EEG studies show that motor experts display distinct automatic response patterns and superior frontal gyrus activity during suppression [26]. These findings highlight the feasibility of monitoring and modulating habitual behavior, though systematic CLBCI application remains scarce.
Recent advances in CLBCIs meet the core needs of habit research, offering single-cell resolution, millisecond precision, multi-region recording, and feedback modulation [8,9,22,24,25]. Compared to earlier tools like tetrodes or calcium imaging that were limited to single regions [2], high-throughput platforms such as the Neuroscroll probe enable brain-wide mapping and tracking of behaviorally relevant neurons [9]. Real-time feedback allows dynamic monitoring of circuit reorganization and intervention during behavioral transitions [22,24].
Clinically, CLBCIs offer promising strategies for disorders involving maladaptive habits. Closed-loop deep brain stimulation (DBS) has improved energy efficiency and responsiveness in OCD treatment compared to open-loop systems [22,23,27]. In addition, EEG θ activity predicts neurofeedback efficacy [28], and BCI-based interventions have reduced craving and smoking [29]. The suppression of habitual responses via non-invasive neuromodulation [7] further illustrates their potential for behavior-level modulation.
Nonetheless, challenges remain. Signal degradation due to gliosis [22], limited biocompatibility [8], and difficulties in integrating distributed circuits [22] continue to constrain long-term application. Overcoming these is key to realizing BCI potential in neuroscience and clinical care.
5. Conclusion
Habitual actions emerge from a distributed, dynamically re-weighted cortico-striato-thalamo-limbic network. Rather than reflecting a simple DMS–DLS switch or fixed S-R mapping, habits involve hierarchical control architectures, dopamine-gated microcircuits, and regionally coordinated action “chunks”. Recent advances in high-density neural probes, graph-based decoding, and closed-loop stimulation now enable real-time read-write access to key network nodes, offering new opportunities for both mechanistic investigation and clinical intervention.
This review is limited by its narrative scope and by the scarcity of human intracranial data in current CLBCI research. Future efforts should expand cross-species datasets and examine long-term biocompatibility and ethical considerations.
Emerging directions include the development of minimally invasive CLBCIs capable of targeting single-neuron ensembles across the habit network, the integration of hierarchical control theory with neural decoders to predict state transitions, and personalized interventions to restore goal-directed control in clinical and everyday contexts.
References
[1]. van Elzelingen, W. et al. (2022) ‘Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation’, Current biology, 32(5), pp. 1163-1174.e6. Available at: https: //doi.org/10.1016/j.cub.2021.12.027.
[2]. Lipton, D.M., Gonzales, B.J. and Citri, A. (2019) ‘Dorsal Striatal Circuits for Habits, Compulsions and Addictions’, Frontiers in systems neuroscience, 13, p. 28. Available at: https: //doi.org/10.3389/fnsys.2019.00028.
[3]. Hermsen, S. et al. (2016) ‘Using feedback through digital technology to disrupt and change habitual behavior: A critical review of current literature’, Computers in human behavior, 57, pp. 61–74. Available at: https: //doi.org/10.1016/j.chb.2015.12.023.
[4]. Smith, K.S. and Graybiel, A.M. (2016) ‘Habit formation’, Dialogues in Clinical Neuroscience, 18(1), pp. 33–43. Available at: https: //doi.org/10.31887/dcns.2016.18.1/ksmith.
[5]. Yin, H.H. (2025) ‘Aligning brain and behavior’, Current opinion in behavioral sciences, 62, p. 101487. Available at: https: //doi.org/10.1016/j.cobeha.2025.101487.
[6]. Grillner, S. (2025) ‘How circuits for habits are formed within the basal ganglia’, Proceedings of the National Academy of Sciences - PNAS, 122(13), p. e2423068122. Available at: https: //doi.org/10.1073/pnas.2423068122.
[7]. Michiels, M. et al. (2025) ‘The neural basis of habit formation measured in goal-directed response switching’, bioRxiv [Preprint]. Available at: https: //doi.org/10.1101/2025.03.13.643040.
[8]. Liu, X. et al. (2024) ‘Flexible high-density microelectrode arrays for closed-loop brain–machine interfaces: a review’, Frontiers in neuroscience, 18, p. 1348434. Available at: https: //doi.org/10.3389/fnins.2024.1348434.
[9]. Liu, Y. et al. (2024) ‘A high-density 1, 024-channel probe for brain-wide recordings in non-human primates’, Nature neuroscience, 27(8), pp. 1620–1631. Available at: https: //doi.org/10.1038/s41593-024-01692-6.
[10]. Yin, H.H. and Knowlton, B.J. (2006) ‘The role of the basal ganglia in habit formation’, Nature Reviews Neuroscience, 7(6), pp. 464–476. Available at: https: //doi.org/10.1038/nrn1919.
[11]. Balleine, B.W. and O’Doherty, J.P. (2010) ‘Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action’, Neuropsychopharmacology, 35(1), pp. 48–69. Available at: https: //doi.org/10.1038/npp.2009.131.
[12]. Lee, K. et al. (2023) ‘Anatomical and Functional Comparison of the Caudate Tail in Primates and the Tail of the Striatum in Rodents: Implications for Sensory Information Processing and Habitual Behavior’, Molecules and cells, 46(8), pp. 461–469. Available at: https: //doi.org/10.14348/molcells.2023.0051.
[13]. Yin, H.H. et al. (2005) ‘The role of the dorsomedial striatum in instrumental conditioning’, European Journal of Neuroscience, 22(2), pp. 513–523. Available at: https: //doi.org/10.1111/j.1460-9568.2005.04218.x.
[14]. Oyama, K. et al. (2024) ‘Distinct roles of monkey OFC-subcortical pathways in adaptive behavior’, Nature Communications, 15(1), p. 6487. Available at: https: //doi.org/10.1038/s41467-024-50505-8.
[15]. Smith, K.S. et al. (2012) ‘Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex’, Proceedings of the National Academy of Sciences, 109(46), pp. 18932–18937. Available at: https: //doi.org/10.1073/pnas.1216264109.
[16]. Kruglanski, A.W. and Szumowska, E. (2020) ‘Habitual Behavior Is Goal-Driven’, Perspectives on Psychological Science, 15(5), pp. 1256–1271. Available at: https: //doi.org/10.1177/1745691620917676.
[17]. Barter, J.W. and Yin, H.H. (2021) ‘Achieving natural behavior in a robot using neurally inspired hierarchical perceptual control’, iScience, 24(9), p. 102948. Available at: https: //doi.org/10.1016/j.isci.2021.102948.
[18]. Malvaez, M. (2020) ‘Neural substrates of habit’, Journal of neuroscience research, 98(6), pp. 986–997. Available at: https: //doi.org/10.1002/jnr.24552.
[19]. Peksa, J. and Mamchur, D. (2023) ‘State-of-the-art on brain-computer interface technology’, Sensors (Basel, Switzerland), 23(13), p. 6001. Available at: https: //doi.org/10.3390/s23136001.
[20]. Janjua, T.A.M. et al. (2021) ‘The effect of peripheral high-frequency electrical stimulation on the primary somatosensory cortex in pigs’, IBRO neuroscience reports, 11, pp. 112–118. Available at: https: //doi.org/10.1016/j.ibneur.2021.08.004.
[21]. Olsen, L.K. et al. (2022) ‘Vagus nerve stimulation-induced cognitive enhancement: Hippocampal neuroplasticity in healthy male rats’, Brain stimulation, 15(5), pp. 1101–1110. Available at: https: //doi.org/10.1016/j.brs.2022.08.001.
[22]. Yang, J. et al. (2024) ‘Precise and low-power closed-loop neuromodulation through algorithm-integrated circuit co-design’, Frontiers in neuroscience, 18, p. 1340164. Available at: https: //doi.org/10.3389/fnins.2024.1340164.
[23]. Cuschieri, A., Borg, N. and Zammit, C. (2022) ‘Closed loop deep brain stimulation: A systematic scoping review’, Clinical neurology and neurosurgery, 223, p. 107516. Available at: https: //doi.org/10.1016/j.clineuro.2022.107516.
[24]. Xue, Q. et al. (2024) ‘Graph neural network based on brain inspired forward-forward mechanism for motor imagery classification in brain-computer interfaces’, Frontiers in neuroscience, 18, p. 1309594. Available at: https: //doi.org/10.3389/fnins.2024.1309594.
[25]. Zhang, S. et al. (2016) ‘A silicon based implantable microelectrode array for electrophysiological and dopamine recording from cortex to striatum in the non-human primate brain’, Biosensors & bioelectronics, 85, pp. 53–61. Available at: https: //doi.org/10.1016/j.bios.2016.04.087.
[26]. He, M., Wen, W. and Qi, C. (2023) ‘Neural Dynamic Underlying Coordination Process between Habitual and Goal-Directed Behavior’, bioRxiv [Preprint]. Available at: https: //doi.org/10.1101/2023.03.16.533062.
[27]. Groppa, S. et al. (2024) ‘Perspectives of Implementation of Closed-Loop Deep Brain Stimulation: From Neurological to Psychiatric Disorders’, Stereotactic and functional neurosurgery, 102(1), pp. 40–54. Available at: https: //doi.org/10.1159/000535114.
[28]. Meng, Q. et al. (2023) ‘Resting-state electroencephalography theta predicts neurofeedback treatment 4-month follow-up response in nicotine addiction’, General psychiatry, 36(4), p. e101091. Available at: https: //doi.org/10.1136/gpsych-2023-101091.
[29]. Bu, J. et al. (2021) ‘BCI-Based Neurofeedback Training for Quitting Smoking’, in Brain-Computer Interface Research. Switzerland: Springer International Publishing AG, pp. 13–23. Available at: https: //doi.org/10.1007/978-3-030-60460-8_2.
Cite this article
Zhang,Y. (2025). Neural Mechanisms of Habitual Behavior and the Potential Applications of BCIs. Theoretical and Natural Science,126,54-63.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. van Elzelingen, W. et al. (2022) ‘Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation’, Current biology, 32(5), pp. 1163-1174.e6. Available at: https: //doi.org/10.1016/j.cub.2021.12.027.
[2]. Lipton, D.M., Gonzales, B.J. and Citri, A. (2019) ‘Dorsal Striatal Circuits for Habits, Compulsions and Addictions’, Frontiers in systems neuroscience, 13, p. 28. Available at: https: //doi.org/10.3389/fnsys.2019.00028.
[3]. Hermsen, S. et al. (2016) ‘Using feedback through digital technology to disrupt and change habitual behavior: A critical review of current literature’, Computers in human behavior, 57, pp. 61–74. Available at: https: //doi.org/10.1016/j.chb.2015.12.023.
[4]. Smith, K.S. and Graybiel, A.M. (2016) ‘Habit formation’, Dialogues in Clinical Neuroscience, 18(1), pp. 33–43. Available at: https: //doi.org/10.31887/dcns.2016.18.1/ksmith.
[5]. Yin, H.H. (2025) ‘Aligning brain and behavior’, Current opinion in behavioral sciences, 62, p. 101487. Available at: https: //doi.org/10.1016/j.cobeha.2025.101487.
[6]. Grillner, S. (2025) ‘How circuits for habits are formed within the basal ganglia’, Proceedings of the National Academy of Sciences - PNAS, 122(13), p. e2423068122. Available at: https: //doi.org/10.1073/pnas.2423068122.
[7]. Michiels, M. et al. (2025) ‘The neural basis of habit formation measured in goal-directed response switching’, bioRxiv [Preprint]. Available at: https: //doi.org/10.1101/2025.03.13.643040.
[8]. Liu, X. et al. (2024) ‘Flexible high-density microelectrode arrays for closed-loop brain–machine interfaces: a review’, Frontiers in neuroscience, 18, p. 1348434. Available at: https: //doi.org/10.3389/fnins.2024.1348434.
[9]. Liu, Y. et al. (2024) ‘A high-density 1, 024-channel probe for brain-wide recordings in non-human primates’, Nature neuroscience, 27(8), pp. 1620–1631. Available at: https: //doi.org/10.1038/s41593-024-01692-6.
[10]. Yin, H.H. and Knowlton, B.J. (2006) ‘The role of the basal ganglia in habit formation’, Nature Reviews Neuroscience, 7(6), pp. 464–476. Available at: https: //doi.org/10.1038/nrn1919.
[11]. Balleine, B.W. and O’Doherty, J.P. (2010) ‘Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action’, Neuropsychopharmacology, 35(1), pp. 48–69. Available at: https: //doi.org/10.1038/npp.2009.131.
[12]. Lee, K. et al. (2023) ‘Anatomical and Functional Comparison of the Caudate Tail in Primates and the Tail of the Striatum in Rodents: Implications for Sensory Information Processing and Habitual Behavior’, Molecules and cells, 46(8), pp. 461–469. Available at: https: //doi.org/10.14348/molcells.2023.0051.
[13]. Yin, H.H. et al. (2005) ‘The role of the dorsomedial striatum in instrumental conditioning’, European Journal of Neuroscience, 22(2), pp. 513–523. Available at: https: //doi.org/10.1111/j.1460-9568.2005.04218.x.
[14]. Oyama, K. et al. (2024) ‘Distinct roles of monkey OFC-subcortical pathways in adaptive behavior’, Nature Communications, 15(1), p. 6487. Available at: https: //doi.org/10.1038/s41467-024-50505-8.
[15]. Smith, K.S. et al. (2012) ‘Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex’, Proceedings of the National Academy of Sciences, 109(46), pp. 18932–18937. Available at: https: //doi.org/10.1073/pnas.1216264109.
[16]. Kruglanski, A.W. and Szumowska, E. (2020) ‘Habitual Behavior Is Goal-Driven’, Perspectives on Psychological Science, 15(5), pp. 1256–1271. Available at: https: //doi.org/10.1177/1745691620917676.
[17]. Barter, J.W. and Yin, H.H. (2021) ‘Achieving natural behavior in a robot using neurally inspired hierarchical perceptual control’, iScience, 24(9), p. 102948. Available at: https: //doi.org/10.1016/j.isci.2021.102948.
[18]. Malvaez, M. (2020) ‘Neural substrates of habit’, Journal of neuroscience research, 98(6), pp. 986–997. Available at: https: //doi.org/10.1002/jnr.24552.
[19]. Peksa, J. and Mamchur, D. (2023) ‘State-of-the-art on brain-computer interface technology’, Sensors (Basel, Switzerland), 23(13), p. 6001. Available at: https: //doi.org/10.3390/s23136001.
[20]. Janjua, T.A.M. et al. (2021) ‘The effect of peripheral high-frequency electrical stimulation on the primary somatosensory cortex in pigs’, IBRO neuroscience reports, 11, pp. 112–118. Available at: https: //doi.org/10.1016/j.ibneur.2021.08.004.
[21]. Olsen, L.K. et al. (2022) ‘Vagus nerve stimulation-induced cognitive enhancement: Hippocampal neuroplasticity in healthy male rats’, Brain stimulation, 15(5), pp. 1101–1110. Available at: https: //doi.org/10.1016/j.brs.2022.08.001.
[22]. Yang, J. et al. (2024) ‘Precise and low-power closed-loop neuromodulation through algorithm-integrated circuit co-design’, Frontiers in neuroscience, 18, p. 1340164. Available at: https: //doi.org/10.3389/fnins.2024.1340164.
[23]. Cuschieri, A., Borg, N. and Zammit, C. (2022) ‘Closed loop deep brain stimulation: A systematic scoping review’, Clinical neurology and neurosurgery, 223, p. 107516. Available at: https: //doi.org/10.1016/j.clineuro.2022.107516.
[24]. Xue, Q. et al. (2024) ‘Graph neural network based on brain inspired forward-forward mechanism for motor imagery classification in brain-computer interfaces’, Frontiers in neuroscience, 18, p. 1309594. Available at: https: //doi.org/10.3389/fnins.2024.1309594.
[25]. Zhang, S. et al. (2016) ‘A silicon based implantable microelectrode array for electrophysiological and dopamine recording from cortex to striatum in the non-human primate brain’, Biosensors & bioelectronics, 85, pp. 53–61. Available at: https: //doi.org/10.1016/j.bios.2016.04.087.
[26]. He, M., Wen, W. and Qi, C. (2023) ‘Neural Dynamic Underlying Coordination Process between Habitual and Goal-Directed Behavior’, bioRxiv [Preprint]. Available at: https: //doi.org/10.1101/2023.03.16.533062.
[27]. Groppa, S. et al. (2024) ‘Perspectives of Implementation of Closed-Loop Deep Brain Stimulation: From Neurological to Psychiatric Disorders’, Stereotactic and functional neurosurgery, 102(1), pp. 40–54. Available at: https: //doi.org/10.1159/000535114.
[28]. Meng, Q. et al. (2023) ‘Resting-state electroencephalography theta predicts neurofeedback treatment 4-month follow-up response in nicotine addiction’, General psychiatry, 36(4), p. e101091. Available at: https: //doi.org/10.1136/gpsych-2023-101091.
[29]. Bu, J. et al. (2021) ‘BCI-Based Neurofeedback Training for Quitting Smoking’, in Brain-Computer Interface Research. Switzerland: Springer International Publishing AG, pp. 13–23. Available at: https: //doi.org/10.1007/978-3-030-60460-8_2.









