

Volume 76
Published on March 2025Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
Purpose: MHCC97-L liver cancer cells, as one of the common liver cancer cells, have 40% metastasis rate of lung cancer. As a commonly used and widely used drug for the treatment of various cancers on the market, doxorubicin has also shown significant effects in treating MHCC97-L cells in previous studies. Meanwhile, research has also shown that ginsenoside Rg3 can play a significant role in the treatment of MHCC97-L liver cancer cells. This study explores the therapeutic effect of the combination of ginsenoside Rg3 and doxorubicin on MHCC97-L liver cancer cells, in both vitro and vivo. Methods: The experiment will use MHCC97-L human liver cancer cell and xenograft mouse model. The cell proliferation is measured by using the CCK-8 detection method and the tumor inhibition rate of the drug in vivo experiments can be calculated formula. Possible results: There are 3 most possible results: (1)The combination of doxorubicin and ginsenoside Rg3 has stronger inhibitory effect than doxorubicin only on MHCC97-L cells, both in vitro and in vivo; (2) The combination of doxorubicin and ginsenoside Rg3 has a strong inhibitory effect in vitro experiments, but the effect is not significant in vivo experiments; (3) The combination of doxorubicin and ginsenoside Rg3 has similar inhibitory effect than doxorubicin only on MHCC97-L cells, both in vitro and in vivo. Conclusion: Our research results will provide important information for the clinical treatment of chemotherapy for lung cancer. Future research should focus on improving drug interactions in vivo and drug metabolism in vivo.

 View pdf
View pdf


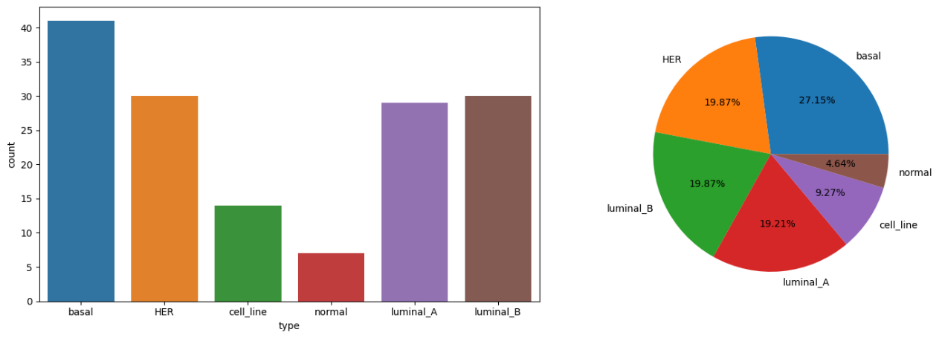
The volatility of global energy markets, particularly electricity prices, plays a crucial role in influencing international economic activities. In the era of big data, machine learning has revolutionized the field of cancer research, particularly in analyzing gene expression data. This study explores the application of machine learning models to the GSE45827 dataset, which contains breast cancer gene expression profiles. With over 54,000 genes and 151 samples categorized into six classes, the dataset presents a high-dimensional challenge that is addressed using dimensionality reduction techniques such as Principal Component Analysis (PCA) and t-distributed Stochastic Neighbor Embedding (t-SNE). The PCA method proved most effective in retaining the critical features of the data in lower dimensions, allowing for clearer visualization and enhanced model performance. The reduced dataset was then classified using the eXtreme Gradient Boosting (XGBoost) model, achieving promising multi-class classification results. The model demonstrated high precision, recall, and F1-scores across several classes, particularly exc elling in classes 1, 2, and 5. However, certain classes, such as 0 and 4, exhibited lower recall, highlighting areas for further refinement. The integration of PCA and XGBoost not only improved the interpretability and computational efficiency of the model but also contributed to the accurate identification of breast cancer subtypes, emphasizing the importance of machine learning in cancer diagnosis and treatment.

 View pdf
View pdf


More than 100 years ago, Paul Ehrlich, the father of immunology, first proposed the concept of “magic bullets”, i.e., the concept of selectively delivering toxic drugs to target cells while avoiding damage to normal cells in the human body. ADCs, as a new type of anticancer drug, are formed by coupling small molecule cytotoxins with monoclonal antibody molecules through linker molecules. As a new type of anticancer drug, ADC couples a small molecule cytotoxin with a monoclonal antibody molecule through a linker molecule, and the drug formed by the combination is targeted and lethal, leading the new era of targeted cancer therapy. In this review, the current development and outlook of ADCs is comprehensively reviewed, ADCs and ongoing R&D pipelines in the global and Chinese markets are analyzed,Various ADC platforms and technologies pertinent to distinct pharmaceutical and biotech firms are emphasized, alongside an exploration of emerging technologies, potential advancements for next-generation ADCs, and avenues for clinical research.

 View pdf
View pdf


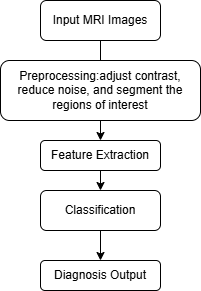
Colorectal cancer (CRC) is one of the most prevalent malignant tumours worldwide, primarily developing on the inner wall of the rectum [1, 2]. Its incidence is closely associated with dietary habits, genetic factors and environmental influences [3]. While early-stage CRC may be asymptomatic, disease progression often leads to abdominal pain, haematochezia, weight loss and fatigue [4]. Consequently, accurate and early diagnosis of CRC is crucial for improving patient survival rates and quality of life.This study aims to comprehensively evaluate and compare different diagnostic methods for colorectal cancer, with a particular focus on the emerging field of AI-enhanced MRI techniques. We hypothesise that the combination of MRI and AI technologies will demonstrate superior diagnostic accuracy and efficiency compared to traditional methods, potentially revolutionising the approach to CRC diagnosis.

 View pdf
View pdf


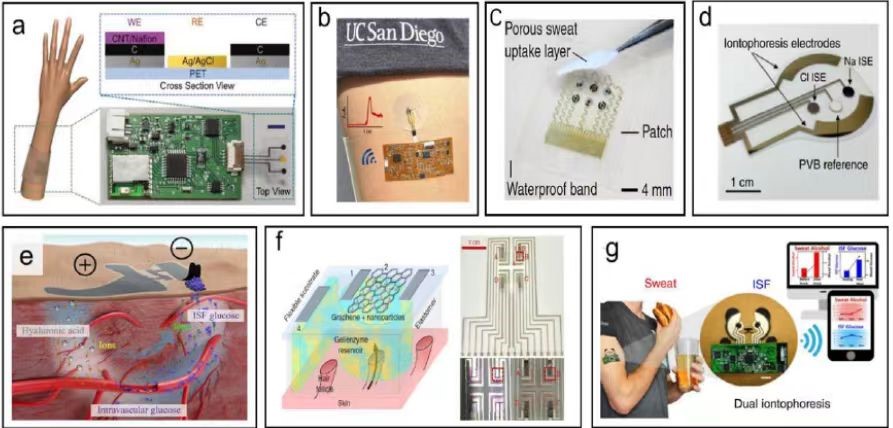
With the rapid development of the Internet of things and medical technology, wearable electrochemical sensors, as an innovative device integrating biotechnology and electronic information technology, have received extensive attention and research in recent years. Compared with other vital signs, the metabolic substances produced in body fluids can be relatively easily collected non-invasively, which has great potential in health diagnosis, exercise monitoring and other aspects. Exercise health monitoring is a major application area for indicator monitoring of sweat parameters [1]. The most commonly used technology is based on electrochemical principles. This paper will elaborate on the relevant progress of the body fluid sampling method and summarize it based on the electrochemical source. The evaluation of wearable sensor performance indicators, the latest progress and related applications, and further exploring its current limitations and future development trends.

 View pdf
View pdf


As the aging population becomes a growing concern in modern society, the incidence of neurodegenerative diseases, characterized by the loss of neurons in the brain and spinal cord, has been steadily rising. An increasing number of individuals are afflicted by these conditions, yet effective solutions remain elusive. Current medical approaches to neurodegenerative diseases are far from ideal, with treatment options primarily focused on attempting targeted therapy through medications. However, these treatments are only capable of alleviating symptoms and cannot provide a cure. Over the past few decades, advancements in science and technology have paved the way for the discovery of novel drugs, antibodies, vaccines, and stem cells, all of which have laid a solid foundation for the successful treatment of neurodegenerative diseases. This paper aims to discuss what neurodegenerative diseases are, the discovery of exosomes, and the relationship between exosomes and the diagnosis and treatment of neurodegenerative diseases.

 View pdf
View pdf


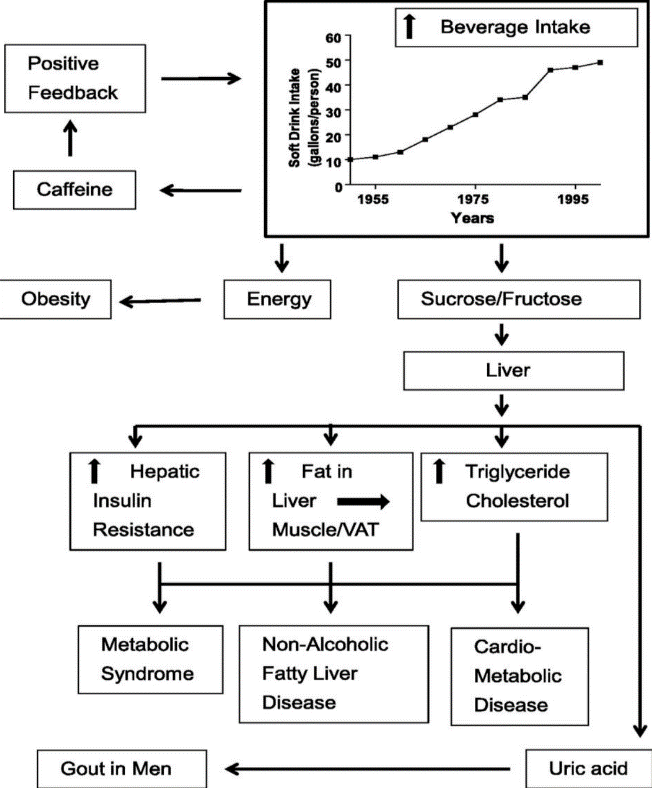
Excessive intake of added sugars and fructose has been associated with leading to adverse health outcomes. This article reviews the effects of sugar intake in a variety of ways, categorized as increased body weight fat, fatty liver (NAFLD), and cardiovascular-related diseases in humans. The analysis showed that decreasing consumption of sugar and sugar products significantly reduced weight, whereas increasing consumption of sugar and sugar products, especially from sugary beverages, led to weight gain. In addition, excessive intake of high sugar is associated with the accumulation of liver fat, a key factor contributing to the increased incidence of fatty liver. The findings highlight the key role of fructose, especially from high fructose corn syrup, in promoting metabolic dysfunction. In conclusion, reducing intake of added sugars is critical to preventing chronic disease and improving public health. Subsequent studies should explore individual differences in response to sugar metabolism and the long-term effects of interventions for small intakes of sugar.

 View pdf
View pdf


Tuberculosis (TB) is a major global infectious disease. The challenges faced in current treatment often include the development of drug resistance, long treatment periods and serious side effects. At the same time, Nanotechnology offers innovative solutions to enhance TB treatment. With the help of nanoparticles such as liposomes, polymeric nanoparticles and inorganic nanoparticles, the drug delivery system can be improved, which leads to higher concentrations of drugs at the site of infection. In this way, side effects are minimized and treatment time is potentially shortened.

 View pdf
View pdf


Background: Alzheimer's disease (AD) is a neurodegenerative disease characterized by abnormal accumulation of Aβ and neuroinflammatory responses. Microglia are key immune cells in the central nervous system and have important regulatory functions in the control of neuroinflammation and neurodegenerative diseases such as Alzheimer's disease. Methods: Microglia have both neuroprotective and neurotoxic effects, but the mechanism by which they protect or damage the nerve remains unclear. Here, I used an in vitro cell culture approach to investigate the role of microglia in neuroinflammation and its regulation of amyloid-β peptides. The same methods were used to investigate the effect of cytokines on microglial polarization and the clearance of amyloid-β. Expected conclusion: Cytokines such as IL-4 and TGF-β can promote the polarization of microglia to M2 direction and enhance its anti-inflammatory effect. Pro-inflammatory factors can induce its polarization to M1 direction and aggravate the degree of neuroinflammation. Therefore, microglia can not only clear amyloid beta, but also damage the nerve and accelerate the process of Alzheimer's disease. By studying this mechanism, I will find ways to promote the large-scale conversion of microglia to M2, which will provide new directions for Alzheimer's disease research and help develop new treatments.

 View pdf
View pdf


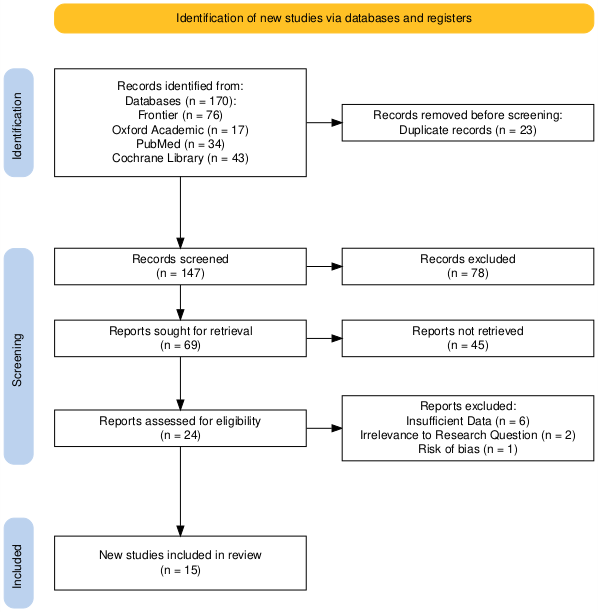
Glucagon-Like Peptide-1 Receptor Agonist (GLP-1) has caught emerging attention in the medical and scientific world as a cure for many obesities, insulin resistance, and diabetically related illnesses [1]. Over the past years, there are projections and few experiment made upon GLP-1’s effect on Polycystic ovary syndrome (PCOS), a hormonal imbalance that affects a woman's ovaries and doesn’t have a cure now. This meta-analysis aims to evaluate the effect of GLP-1 RAs on PCOS by reviewing and synthesizing data from randomized controlled trials (RCTs) and clinical trials. We analyzed 14 studies with 669 participants published between 2008 and 2023 across four countries. A random-effects model was used to compare the standardized mean difference (SMD) in body mass index (BMI) and other clinical parameters between treatment and control groups. Our outcome showed no significant reduction in BMI with GLP-1 RAs compared to placebo in PCOS patients (SMD -3.13, 95% CI: -6.44 to 0.17), with high heterogeneity (I² = 96%). Secondary outcomes yields that no significant differences were found in weight reduction, HOMA-IR scores, or hormone levels (testosterone, cholesterol, DHEA) when comparing GLP-1 RAs with metformin (SMD -0.10, 95% CI: -0.48 to 0.28). These findings suggest that GLP-1 RAs do not significantly improve PCOS symptoms. The substantial heterogeneity across studies highlights the need for further research with larger, more standardized sample sizes.

 View pdf
View pdf




