

Volume 90
Published on February 2025Volume title: Proceedings of ICMMGH 2025 Workshop: Computational Modelling in Biology and Medicine
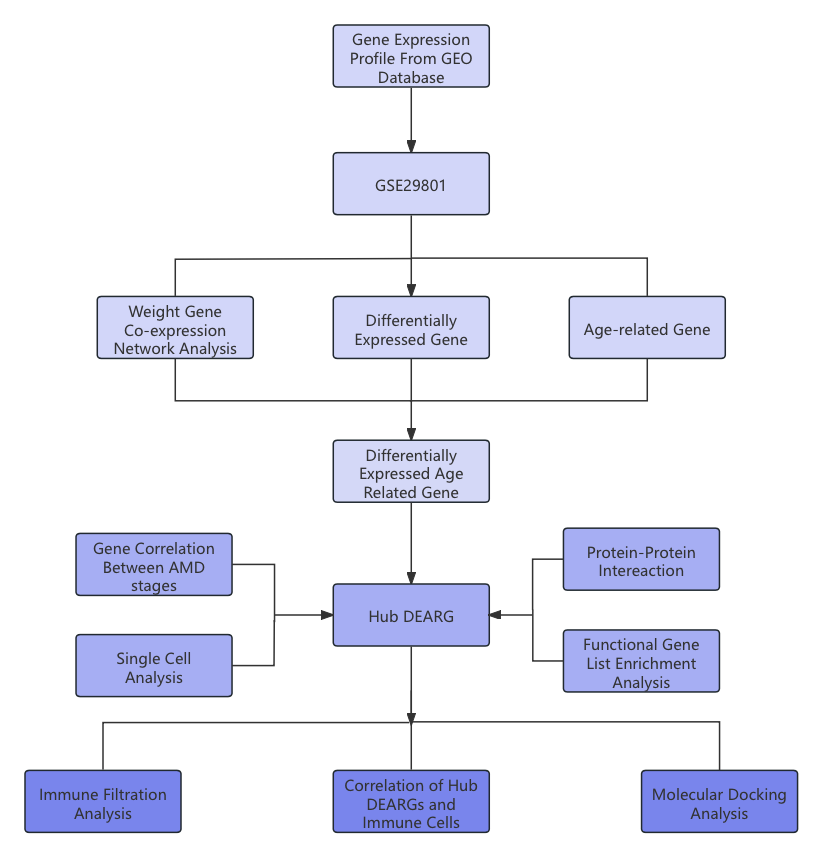
Age-related macular degeneration (AMD) is the leading cause of visual impairment in older adults worldwide and is a condition that causes visual deprivation. There exist two subcategories of this disease with the wet form of this disease being the focus of our study. Using bioinformatic analysis, this research conducted investigations to uncover the genes related to aging that may be biomarkers for the development of AMD. First, we compared the expression levels of samples from AMD/CNV patients and a control group using the GEO microarrays (GSE29801) in order to obtain differentially expressed genes (DEGs). WGCNA, combined with functional enrichment analysis, is utilized to discover and validate the gene module crucial for AMD. Differentially expressed aging-related genes (DEARGs) were identified by overlapping significant gene sets. The subcellular location of hub DEARGs and their corresponding cell subpopulations were determined and predicted using the Geo dataset GSE155288. Pan-cancer analyses were used to confirm those hub DEARGs’ function in other diseases. Moreover, both Protein-Protein Interaction (PPI) and AlphaFold prediction were employed to validate the protein interaction among the key DEARGs. Lastly, a potential target drug was selected, with portions of them validated through drug-protein interactions. In further analysis of our result, the collect gene set of 7 DEARGs was divided into the immune-related group and the non-immune-related group. These groups uncovered two distinct pathways of AMD development, with one triggering inflammatory responses by promoting macrophage proliferation and the other inducing choroidal neovascularization formation due to malfunctioning growth regulator genes

 View pdf
View pdf


RETT syndrome is an X-chromosome-linked neurodevelopmental disorder mainly affecting female children, and its pathogenesis is mainly related to mutations in the Mecp2. In recent years, the role of Mecp2 in neurodevelopment and its relationship with RETT syndrome has become a hot research topic. Current research is focused on the effects of Mecp2 mutations on neuronal function and neural circuits, as well as exploring potential therapeutic approaches in animal models. Despite the results achieved, there are still gaps in the understanding of the precise molecular mechanisms of the Mecp2, gender specificity and the role of non-coding RNAs. This paper analyzed the role of Mecp2 in neurodevelopment and its relationship with Rett syndrome, and explored the molecular mechanisms and potential therapeutic strategies for Mecp2 mutations. It was found that Mecp2 mutations lead to neurological dysfunction by affecting neuronal differentiation and synaptic plasticity. In addition, this paper evaluates the current prospects for gene therapy, noting that adeno-associated viral vectors (AAV) have shown favorable therapeutic effects in animal experiments. This paper provides new insights into understanding the pathogenic mechanism of the Mecp2 in RETT syndrome and provides an important reference for future therapeutic strategies. However, the precise molecular mechanism of the Mecp2 and its gender-specific differences still need further investigation. Future studies could focus on developing safer and more effective gene therapies and exploring the role of non-coding RNAs in RETT syndrome with a view to achieving more comprehensive therapeutic strategies

 View pdf
View pdf


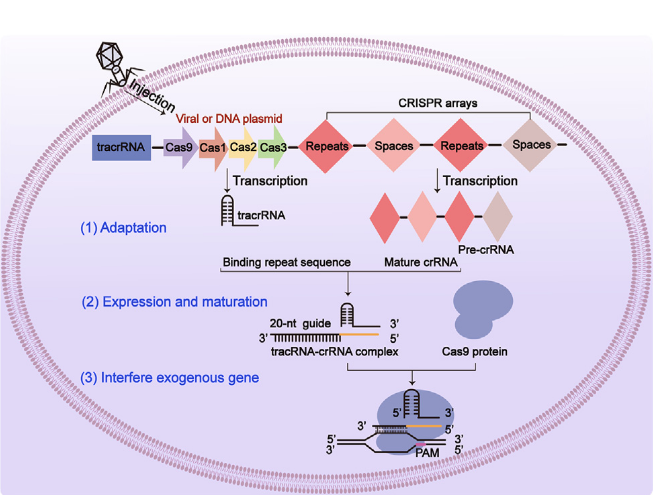
Liver cancer affects a significant number of individuals annually, and it ranks as the third leading cause of cancer-related deaths. In the past decade, significant advances have been brought to provide more options for treating hepatocellular carcinoma (HCC). However, the development of treatment resistance in HCC patients raises challenges for clinicians in managing this malignant tumor. The use of the CRISPR/Cas9 system to identify genes that enhance clinical response in HCC patients lays a solid foundation for personalized treatment. This article summarizes the current understanding of using the CRISPR/Cas9 system in cancer therapy, particularly emphasizing its application to HCC. This review presents examples and relative mechanisms of using CRISPR/Cas9 with immune checkpoint inhibitors to control tumor cell growth and discusses how CRISPR/Cas9 can prevent viral hepatitis from progressing to HCC. This review aims to improve patient prognosis and fundamentally transform the treatment landscape of HCC by reviewing the prospects and current status of CRISPR technology in the treatment of HCC

 View pdf
View pdf


Cancer has been one of the most concerning diseases around the globe, and oncologists have been developing different insightful therapies in the last century. With the shifted focus on immunotherapy, analysing the tumour microenvironment (TME) is crucial for cancer hallmark identification and subsequent antibody-based inhibition. Currently, PD-1/PD-L1 and CTLA-4 immune checkpoints are studied intensively, and there are FDA-approved immune checkpoint inhibitors (ICIs) such as Ipilimumab for anti-CTLA-4. Also, the characteristics of TMEs are expanded to include genomic instability and epigenetic factors. However, the complexities of TMEs disallow oncologists to consider all aspects, and tackling immune checkpoints can lead to unintended consequences. Therefore, this review report aims to investigate the common biochemicals and immune responses within TMEs, and how different types of TMEs have different overexpressions. Based on the chemical interactions in TME, the targetable cancer hallmarks will be investigated, with an inclination toward the fast-growing immune checkpoint inhibitors. It is concluded that cytokine-induced inflammation in TME causes Treg responses and immune escape by tumour cells, which can be categorised into different types. Also, immune checkpoint inhibitors show promising results against solid or liquid tumours when used in the right conditions. Overall, this report emphasises the diversity and informs the key characteristics of TMEs; it reminds the medical field that immune checkpoint inhibitors may not always bring a better prognosis. A thorough understanding of TME is important for future research developments. In the future, there could be more focus on combination therapies on immune checkpoints

 View pdf
View pdf


As an important part of modern biotechnology, genetic engineering is widely used in fields such as disease treatment, agricultural improvement, and environmental protection. Gene sequencing technology, especially next-generation sequencing technology, provides a powerful tool for studying biological genetic information. However, with the rapid growth of genomic data, how to efficiently and accurately analyze and apply this huge data has become a major challenge facing genetic engineering. In recent years, artificial intelligence (AI) technology, especially deep learning, has been widely used in the automated processing of large-scale data and has shown great potential in genetic engineering. AI technology not only shows advantages in gene editing optimization, genetic variation detection and genome association analysis, but also significantly improves the efficiency and accuracy of genetic data analysis. Although AI has brought many conveniences in genetic engineering, challenges such as technology transparency, data quality issues, and ethics and privacy protection still need to be solved. This article explores the application of artificial intelligence in genetic engineering sequencing and data analysis, analyzes how AI can improve the efficiency and accuracy of genetic data analysis, and discusses the potential contribution of AI in gene editing and precision medicine. As AI continues to develop, it is expected to play an increasingly important role in fields such as genomics, gene editing, and precision medicine, and provide more effective strategies for future disease treatment

 View pdf
View pdf


Pancreatic ductal adenocarcinoma (PDAC) is a highly invasive and highly lethal malignant tumor. Due to the difficulty in early diagnosis and limited treatment options, the 5-year survival rate is less than 10%. Traditional treatments such as surgery, chemotherapy, and radiotherapy have limited effects on PDAC, which is mainly attributed to its unique tumor microenvironment (TME), common gene mutations (such as KRAS, and TP53), and immunosuppressive properties. In recent years, with the advancement of molecular biology and genomics technologies, the molecular mechanisms of PDAC have been studied more deeply, revealing the gene mutations and regulatory networks associated with its pathogenesis. In particular, KRAS mutations have become an important research direction for targeted therapy. However, the effect of immunotherapy in PDAC is limited by the immune escape characteristics of TME. This article systematically summarizes the key gene mutations in PDAC and their regulatory mechanisms, including epigenetic regulation, microRNA, histone modification and other influencing factors. CRISPR-Cas9 shows great potential in correcting gene mutations and reshaping TME. In addition, combining immunotherapy with targeted drug delivery (such as nanotechnology) can improve the precision of treatment and reduce side effects. These advances provide a comprehensive reference for future personalized and effective treatment options for PDAC

 View pdf
View pdf


Neurodegenerative diseases (NDDs) include Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD), which are defined by the progressive deterioration of neurons in the central nervous system and functional decline. The pathogenesis of these diseases is complex and there is currently no effective treatment. For example, the failure rate of clinical trials for AD treatment strategies is as high as 99.5%. Research indicates that elements such mitochondrial malfunction, oxidative stress, excitotoxicity, inflammation, and apoptosis are closely related to the onset of NDDs, especially mitochondrial dysfunction, which is considered to be one of the core mechanisms of NDDs. Mitochondria are not only the main production site of ATP, but also participate in key processes such as metabolite synthesis and reactive oxygen generation. Due to the high energy demand of brain neurons and their high dependence on mitochondrial function, abnormal mitochondrial function may lead to serious neuronal structural and functional disorders. In addition, neurons also need to maintain local activities such as synaptic transmission and axonal transport through the precise transport and distribution of mitochondria. Therefore, abnormalities in mitochondrial dynamics and function may become early characteristics and potential pathogenic factors of NDDs. By reviewing the mechanism of mitochondrial abnormalities in AD, PD, and HD and their potential therapeutic strategies.This article seeks to investigate mitochondria as a prospective target for the prevention, early detection, and treatment of NDD

 View pdf
View pdf


Intestinal cancers are always hazardous due to its high morbidity and mortality. Currently, it is known that an error in the Wnt/β-catenin pathway is an essential cause of intestinal cancers. After the inhibitors and markers involved in it are especially studied, targeted therapy has already been considered by the scientists. However, there is only overall understanding in Wnt/β-catenin pathway with many detailed mechanisms uncleared, thus no treatments can be put into practice. This article analyses proteins and factors which have the potential of being used as targets of targeted therapy. Besides, essential inhibitors, such as NEDD4 and E7386, of the Wnt/β-catenin pathway and how lack of them lead to oncogenesis are introduced. Furthermore, the possibility and examples of targeted medicine is discussed. This review offers a wider view of the entire mechanism of intestinal cancers spurred by Wnt/β-catenin pathways. The article encouraged further research in variable stages, and new breakthrough is purposed for therapy based on the Wnt/β-catenin pathway

 View pdf
View pdf


Stem cell research has advanced rapidly, offering promising treatments for refractory diseases due to their unique capabilities for self-renewal and pluripotent differentiation. Stem cells play pivotal roles in treating genetic disorders, neurodegenerative diseases (NDDs), cardiovascular conditions, and cancer. In genetic diseases, combining stem cells with gene-editing tools like CRISPR-Cas9 enables precise correction of pathogenic genes, while healthy stem cells repair tissue by replacing diseased cells. For NDDs, iPSCs can differentiate into dopaminergic neurons to replace damaged brain cells and enhance neural regeneration. In cardiovascular diseases, they promote myocardial and vascular repair. In cancer, stem cells boost anti-tumor immunity and deliver drugs directly to tumor sites, improving treatment efficacy. Despite these breakthroughs, challenges persist. High-quality stem cell production is limited, and controlling differentiation to prevent tumorigenesis remains critical. Allogeneic transplants risk immune rejection, and using embryonic stem cells raises ethical concerns. Regulatory frameworks and clinical standards are needed to ensure safety and efficacy, alongside addressing ethical and patient rights issues. With continued innovation, stem cell therapy is going to revolutionize medicine, offering novel methods for complex diseases and improving global health

 View pdf
View pdf


With global climate change and increasing environmental pollution, plants are facing increasingly severe abiotic stresses, such as drought, salinization and heavy metal pollution. These stresses not only affect plant growth and development, but also pose a threat to agricultural production and ecosystem stability. In order to adapt to these unfavorable environments, plants have evolved a series of complex response mechanisms, among which epigenetic regulation, as an important means of regulation, has gradually attracted the attention of researchers. By modifying gene expression without modifying the DNA sequence, epigenetic control uses a variety of techniques, including DNA methylation, histone modification, and non-coding RNAs (ncRNA). These mechanisms are crucial for plant responses to abiotic stresses and can significantly enhance plant resistance and adaptive capabilities. DNA methylation can enhance plant resistance by controlling the expression of stress response-related genes; histone modification can regulate plant physiological responses by altering the structure of chromatin and affecting the accessibility of genes; and ncRNAs, especially microRNAs and siRNAs, can regulate plant physiological responses by targeting the expression of stress response-related genes. In-depth study of the role of plant epigenetic regulation in abiotic stress response is of great theoretical and practical significance for improving crop resistance and ensuring food security

 View pdf
View pdf




