

Volume 126
Published on July 2025Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
Type 1 diabetes (T1D) is a chronic disease characterized by autoimmune destruction of pancreatic β cells, resulting in a lack of insulin secretion. Current treatments mainly rely on exogenous insulin supply. Although automated insulin infusion systems can effectively control blood sugar, they cannot reverse immunopathology or restore endogenous insulin secretion. This article divides emerging treatments for T1D into three categories: immune regulation, immune tolerance induction, and β cell regeneration and replacement. Immunomodulatory methods such as anti-CD3 antibodies (such as Teplizumab) and mesenchymal stem cell (MSC) infusion have shown some promise in preserving β cell function; tolerance induction therapy is based on autoantigens, such as insulin and GAD65 vaccines, but the efficacy is greatly affected by individual HLA differences. Emerging CAR-Tregs have higher specificity and potential. Regenerative and replacement therapies such as hematopoietic stem cell transplantation and islet-like cell transplantation have significant efficacy, but the need for immunosuppression remains their main challenge. At present, most treatments are still in the clinical trial stage. In the future, we can try to combine therapies with multiple mechanisms in order to achieve a more lasting and fundamental therapeutic effect.

 View pdf
View pdf


Due to their high specificity and low toxicity, antibody drugs have emerged as a key therapeutic approach for the treatment of cancer and autoimmune diseases. In recent years, artificial intelligence (AI), as the driving force of the Fourth Industrial Revolution, has been reshaping the paradigm of antibody drug development. This paper systematically reviews the application of AI throughout the entire development pipeline of antibody drugs, including target discovery and validation, antibody design and optimization, experimental design and functional verification, as well as preclinical and clinical research. Although AI holds great promise in the field of antibody development, it still faces a number of challenges, such as inconsistent data quality, poor model interpretability, and unresolved issues in technology ethics. The integration and advancement of generative AI and cutting-edge computational technologies are expected to accelerate the transformation of antibody drug development toward a more intelligent and precise direction.

 View pdf
View pdf


Cancer, despite decades of focused research and treatment development, still stands as one of the most stubborn threats to human health. Common treatments such as surgery, radiation, chemotherapy are effective, but they often fall short. Side effects can be severe, and results vary from patient to patient. More recent strategies, like immunotherapy or targeted drugs, seem promising but don't work across the board. What this paper tries to do is look at an alternative—combining drugs in a way that zeroes in on how cancer cells work differently from normal ones. Tumors grow in ways they shouldn't,mess with metabolism, resist cell death, and even fool the immune system. That opens the door to some overlooked treatments—things that change how cancer cells make energy, or that ramp up oxidative stress. It even includes tools like DNA origami, which sounds strange but lets drugs be delivered more precisely. The point isn’tto find one cure, but to build smarter combinations that match the disease’s complexity and do less harm in the process.

 View pdf
View pdf


Global population aging is a hot topic in the 21stcentury, and it drives demand for anti-aging interventions. However, there are no proven anti-aging drugs available, and there are also ethical and practical challenges in testing anti-aging compounds in humans. In this case, Caenorhabditis elegans (C.elegans) serves as a powerful model to study aging due to genetic similarities with humans and short lifespans. In this study, we tested the effect of four compounds (metformin, rapamycin, royal jelly, and rilmenidine) on C. elegans aging. Metformin and rapamycin significantly extended lifespan by 30% and 23%, respectively. Meanwhile, metformin uniquely enhanced the pumping rate, indicating slowed aging. Among the aging-related pathways in C. elegans, the metabolic genes play a vital function. To test the mechanism of metformin in slowing aging, we analyzed the aging-related metabolic genes with RT-qPCR. Metformin was demonstrated to upregulate the expression of the metabolic gene, isocitrate dehydrogenase alpha-1 (idha-1), potentially by elevating the tolerance to oxidative stress. Results highlight idha-1 as a key target for anti-aging interventions.

 View pdf
View pdf


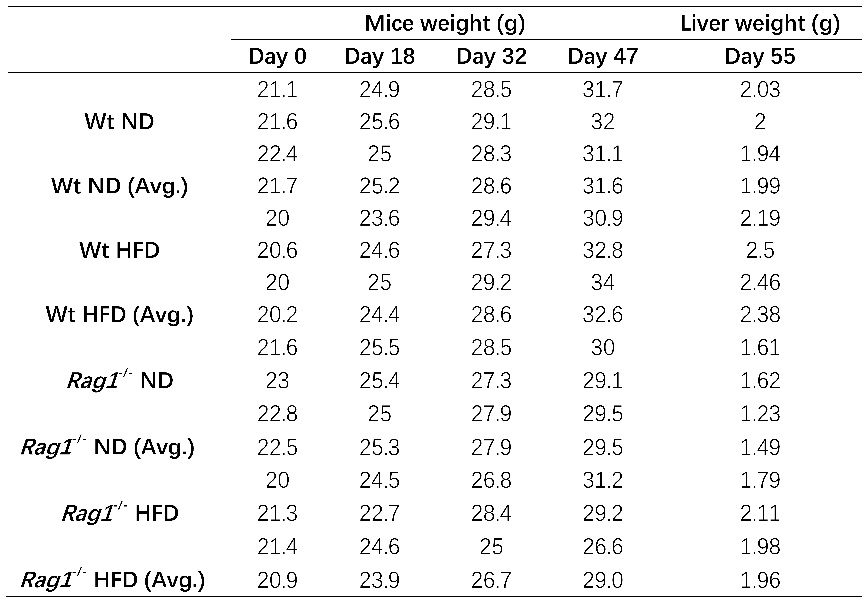
This study investigated the effects of adaptive immune deficiency (achieved by Rag1 gene knockout, Rag1-/-) on metabolic disorders induced by high-fat diet in mice, especially liver changes and apolipoprotein E (ApoE) expression. Wild-type (Wt) C57BL/6J mice and Rag1-/-mice were randomly divided into normal diet group and high-fat diet group and fed for 8 weeks. The results showed that high-fat diet significantly increased the liver weight of Wt and Rag1-/-mice and downregulated the expression of ApoE mRNA in the liver. Although not significant, the increase in body weight and liver weight caused by high-fat diet in Rag1-/-mice was generally lower than that in Wt high-fat diet treatment. The expression of ApoE gene in liver tissue of Rag1-/-mice was lower than that of Wt mice, and high-fat diet further reduced the expression of ApoE gene. These results indicate that mature lymphocytes (T, B cells) have a certain regulatory effect on the expression of ApoE gene in the liver, as well as on metabolic disorders and liver damage induced by high-fat diet, which provides a new approach for targeting the immune system to regulate liver metabolism.

 View pdf
View pdf


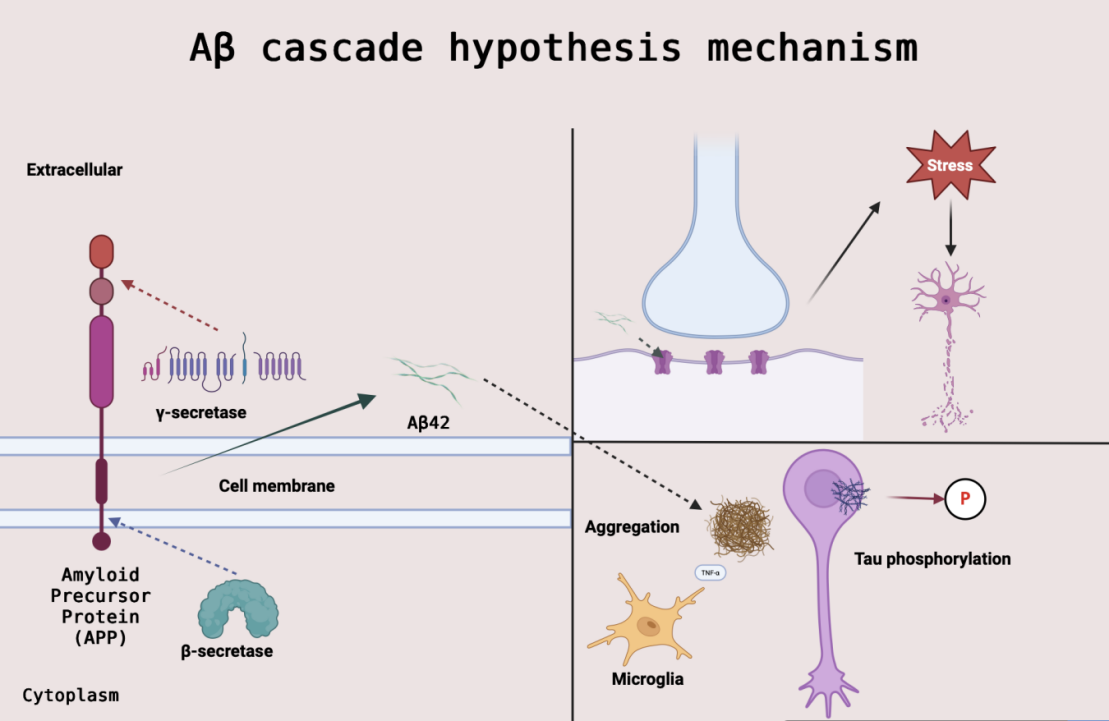
Alzheimer's disease (AD), the world's premier neurodegenerative disease, has long lacked effective disease-modifying therapies for its core pathological mechanisms - β-amyloid (Aβ) deposition and tau protein tangles. In recent years, research breakthroughs have focused on anti-Aβ monoclonal antibodies, among which Lecanemab has become the first disease-modifying drug fully approved by the FDA by targeting soluble Aβ protofibrils, with a significant delay in cognitive decline of 27% (CDR-SB) and a manageable risk of ARIA (11.6%) in Phase III. However, the lack of dynamic monitoring technology, the uncertainty of long-term safety, and the cost-effectiveness paradox remain unresolved. This paper systematically analyses Lecanemab's mechanism of action (selective removal of protofibrils), clinical evidence (dose-response and safety in phase II/III trials), and integrate a biomarker (p-tau217) driven stratification strategy with the ARIA risk management framework. It was found that precision interventions for toxic Aβ subtypes can reshape treatment regimens, but need to be combined with tau-targeted therapies to enhance late-stage efficacy. This review provides three major references for precision therapy in AD: establishing the stratification value of early biomarkers, optimising risk-benefit assessment models, and revealing pathways for combination therapy potentiation. In the future, plasma protofibril detection technology needs to be developed, APOE genotype-oriented ARIA prediction algorithms need to be established, and low-cost delivery systems need to be explored to enhance accessibility.

 View pdf
View pdf


Childhood obesity poses a severe global public health threat, with China facing a projected overweight/obesity rate of 28.0% among children aged over 7 by 2030, adversely impacting both physical and mental health. This paper comprehensively analyzes the intervention effects of jogging on preventing obesity and influencing the growth and development of primary school students. The results of this paper indicate that jogging effectively regulates energy balance, activates aerobic metabolism, significantly burns calories through hormonal regulation, improves insulin sensitivity, reduces fat synthesis and enhances satiety. Physiologically, jogging enhances cardiorespiratory fitness, promotes bone/muscle development and increases bone mineral density. Crucially, jogging can also have significant mental health benefits, reducing anxiety and depression while increasing self-esteem and confidence. In addition, it enhances cognitive functioning, particularly executive control and breadth of attention in the classroom (12%-18% increase). In addition, group jogging activities help develop social adjustment and life skills. This paper identifies major barriers to physical activity (academic stress, lack of safe spaces, and screen time) and proposes multilevel prevention strategies involving school, family, and society, findings that provide strong evidence for the development of effective obesity prevention policies and the promotion of holistic child development.

 View pdf
View pdf


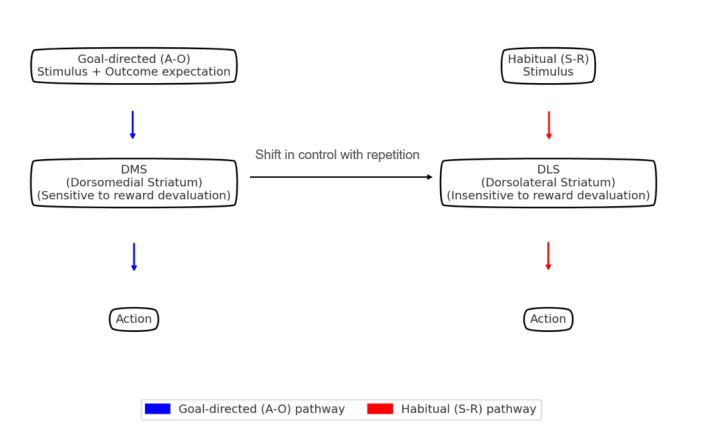
Habits are fundamental to human behavior, enhancing efficiency through repeated procedural responses. However, such automaticity can become maladaptive when behavioral flexibility declines. Dysfunctional habit circuits have been linked to addiction, obsessive-compulsive disorder (OCD), and repetitive behaviors in autism spectrum disorder (ASD). These findings underscore the need for a mechanistic understanding of habit-related neural dynamics and precise interventions. This paper presents a literature review of the neural mechanisms underlying habitual behavior, emphasizing current and emerging applications of brain–computer interfaces (BCIs), particularly closed-loop BCIs (CLBCIs), in the investigation and modulation of habitual control. The review identifies two core points: traditional models tend to oversimplify cortico-striatal dynamics, while newer BCI technologies may support more precise investigation. By discussing how BCIs address longstanding methodological gaps, this review highlights their potential for real-time modulation of habit-related circuits. While challenges remain in signal quality, regional integration, and biocompatibility, BCIs hold clear promise for advancing both neuroscience and clinical intervention.

 View pdf
View pdf


Aortic dissection is one of the diseases that seriously endanger life and health, and the average age of onset in China is lower than the international average age and shows a trend of rejuvenation. This paper provides an in-depth analysis and discussion of postoperative exercise, influencing factors and improvement measures for patients with aortic coarctation. This paper will focus on specific prescription recommendations for exercise interventions and their effects on improving patients' neurological, circulatory, and emotional well-being. The results of this paper show the need to improve the participation and effectiveness of exercise rehabilitation in Chinese patients with type B aortic coarctation while reducing patient resistance to rehabilitation (location, finances, and fear), and suggest a variety of activity recommendations and a range of activity standards. Based on the patients' own rehabilitation assessment, individualized exercise prescription was developed to achieve the rehabilitation goals of improving cardiopulmonary function, attenuating the influence of negative psychological factors, and reducing the incidence of delirium complications. The effectiveness of this is shown in the improvement of patients' post-operational quality of life and the maximization of their mobility recovery.

 View pdf
View pdf


The proliferation of antibiotic-resistant bacterial strains represents a critical threat to contemporary healthcare systems worldwide. This comprehensive analysis investigates the fundamental molecular pathways that enable bacterial resistance while examining innovative therapeutic alternatives to conventional antimicrobial treatments. Our systematic review encompasses key resistance phenomena including active drug efflux, molecular target alterations, enzymatic drug degradation, protective biofilm establishment, and genetic material exchange between species. We further assess novel therapeutic modalities encompassing advanced drug design, biotechnology-based interventions, and ecosystem-wide management strategies. Our findings underscore the necessity for integrated, multisectoral approaches informed by One Health principles to combat this escalating crisis. We particularly highlight the transformative potential of CRISPR-based technologies and nanomedicine platforms in reshaping future antimicrobial treatment paradigms.

 View pdf
View pdf




