

Volume 99
Published on May 2025Volume title: Proceedings of the 5th International Conference on Biological Engineering and Medical Science
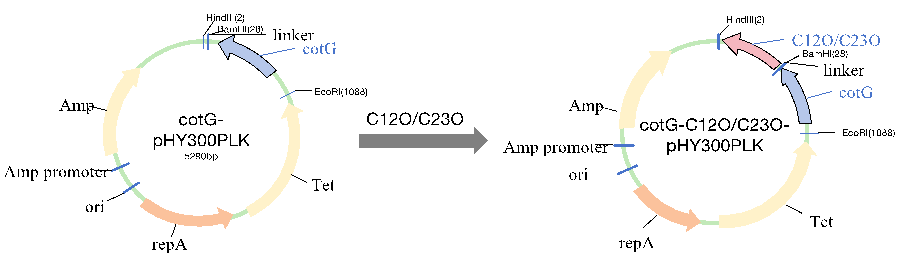
Catechol is a pollutant commonly found in industrial and everyday wastewater, with genotoxic effects that can lead to mutations, DNA fragmentation, and chromosomal aberrations. Traditional physical and chemical degradation methods face challenges such as high costs and secondary pollution, whereas biological degradation offers a cost-effective and environmentally friendly alternative. This study investigates the use of the Bacillus subtilis spore surface display system for catechol degradation, aiming to develop an efficient, low-cost biodegradation method. Recombinant Bacillus subtilis was successfully engineered to display catechol 1,2-dioxygenase (C12O) and catechol 2,3-dioxygenase (C23O) on the spore surface. Immunoblotting and immunofluorescence confirmed protein display and enzyme activity assays demonstrated catechol degradation activity. The displayed enzymes showed stability across a wide pH and temperature range, maintaining activity even under methanol conditions. Compared to purified proteins, the spore-bound enzymes eliminated purification steps, reducing costs and improving degradation efficiency in some conditions. These results suggest that the Bacillus subtilis spore display system has great potential in biocatalysis, offering a novel strategy for environmental pollution remediation.

 View pdf
View pdf


Chronic myelogenous leukemia (CML) and non-small cell lung cancer (NSCLC) are two common malignant tumors. Traditional treatments mainly include chemotherapy, radiotherapy, and bone marrow transplantation for CML. However, the low success rate of donor matching limits the widespread use of bone marrow transplants. The development of tumors is closely related to mutations or overexpression of multiple oncogenes, but their regulatory mechanisms and effective therapeutic strategies are still unclear. In recent years, the rapid development of gene editing technology, especially the CRISPR-Cas9 system, has provided new technical means for screening therapeutic targets for leukemia and lung cancer. This project utilizes the CRISPR-Cas9 system to knock out the MYC gene, which is considered a key oncogene in many human cancers. The first step of this project is to construct MYC gene knockout cell lines for CML and NSCLC, and to evaluate the effects on tumor cell proliferation and differentiation in vitro. It is expected that knocking out the MYC gene will significantly inhibit tumor cell proliferation and activate the expression of differentiation-related genes. This study will help reveal the role of the MYC gene in tumorigenesis and provide important theoretical support and potential clinical application value for targeted therapy of CML and NSCLC, further promoting the development of personalized precision medicine.

 View pdf
View pdf


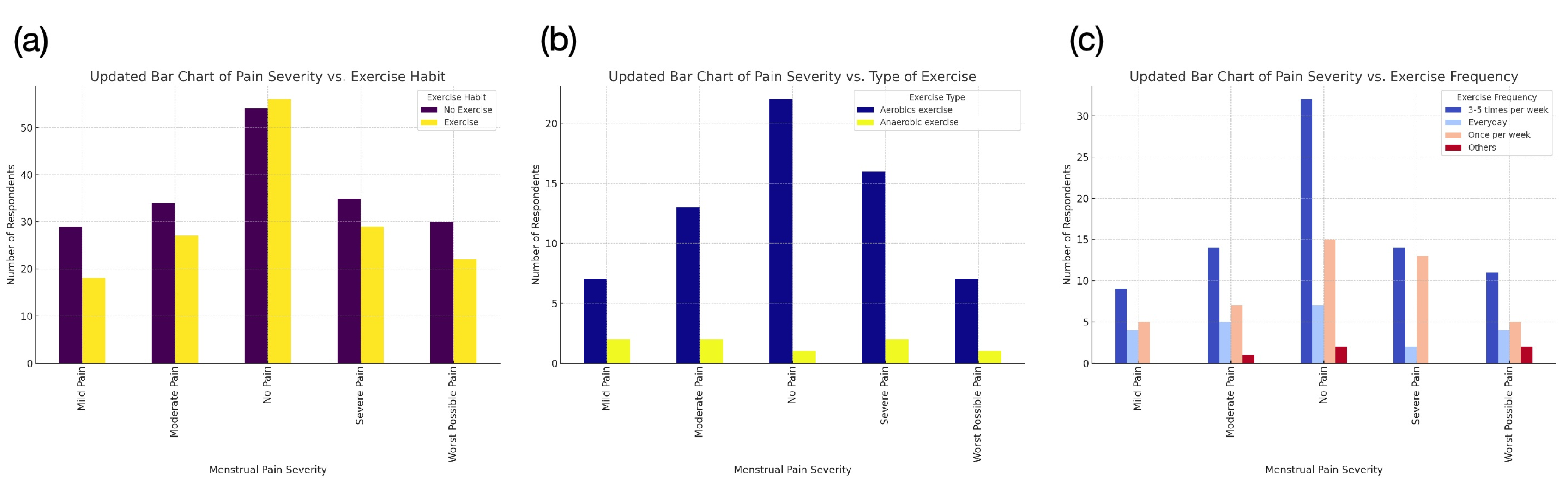
Dysmenorrhea significantly impacts the quality of life and health of many women worldwide, with primary dysmenorrhea being a major component. Primary dysmenorrhea is primarily driven by the excessive production of prostaglandins, which are responsible for regulating uterine contractions and the shedding of the endometrium. While prostaglandins are essential for normal bodily functions, their overproduction during menstruation leads to severe cramps and considerable pain. In this review, we examine the key biological pathways that contribute to dysmenorrhea, along with the mechanisms behind current treatment strategies and their effectiveness. Based on the current understanding of dysmenorrhea’s underlying mechanisms and the insights gained from the reviewing process, we propose potential new drug targets for treatment. Furthermore, we offer comprehensive recommendations for managing dysmenorrhea, combining the results of the further analysis of our previous survey data. We also emphasize the significance of interdisciplinary research, with collaboration across various fields to enhance our understanding and improve the comprehensive management of dysmenorrhea, ultimately benefiting women's health and well-being more effectively.

 View pdf
View pdf


SARS-CoV-2, the causative agent of COVID-19, represents a significant global health challenge. The continuous emergence of variants with immune-evasion capabilities, particularly Delta, Beta, and Omicron, has raised concerns about the long-term efficacy of current vaccines. These variants harbor mutations in the spike protein that alter viral binding affinity and enable escape from vaccine-induced neutralizing antibodies. This research examines vaccine design strategies targeting breakthrough variants, with particular focus on antibody-tolerant functions and mutational adaptability characteristics. We analyze modifications in spike protein configuration and function, especially within the receptor-binding domain (RBD), which primarily contributes to immune escape. Additionally, we evaluate how these mutations impact vaccine development and propose broad-spectrum protection strategies. By integrating insights from molecular evolution, structural biology, and immunology, this study provides a comprehensive framework for understanding SARS-CoV-2 and offers novel perspectives on future vaccine design. Our findings underscore the importance of developing mutation-responsive vaccines and therapeutic approaches to address continuously evolving viral threats.

 View pdf
View pdf


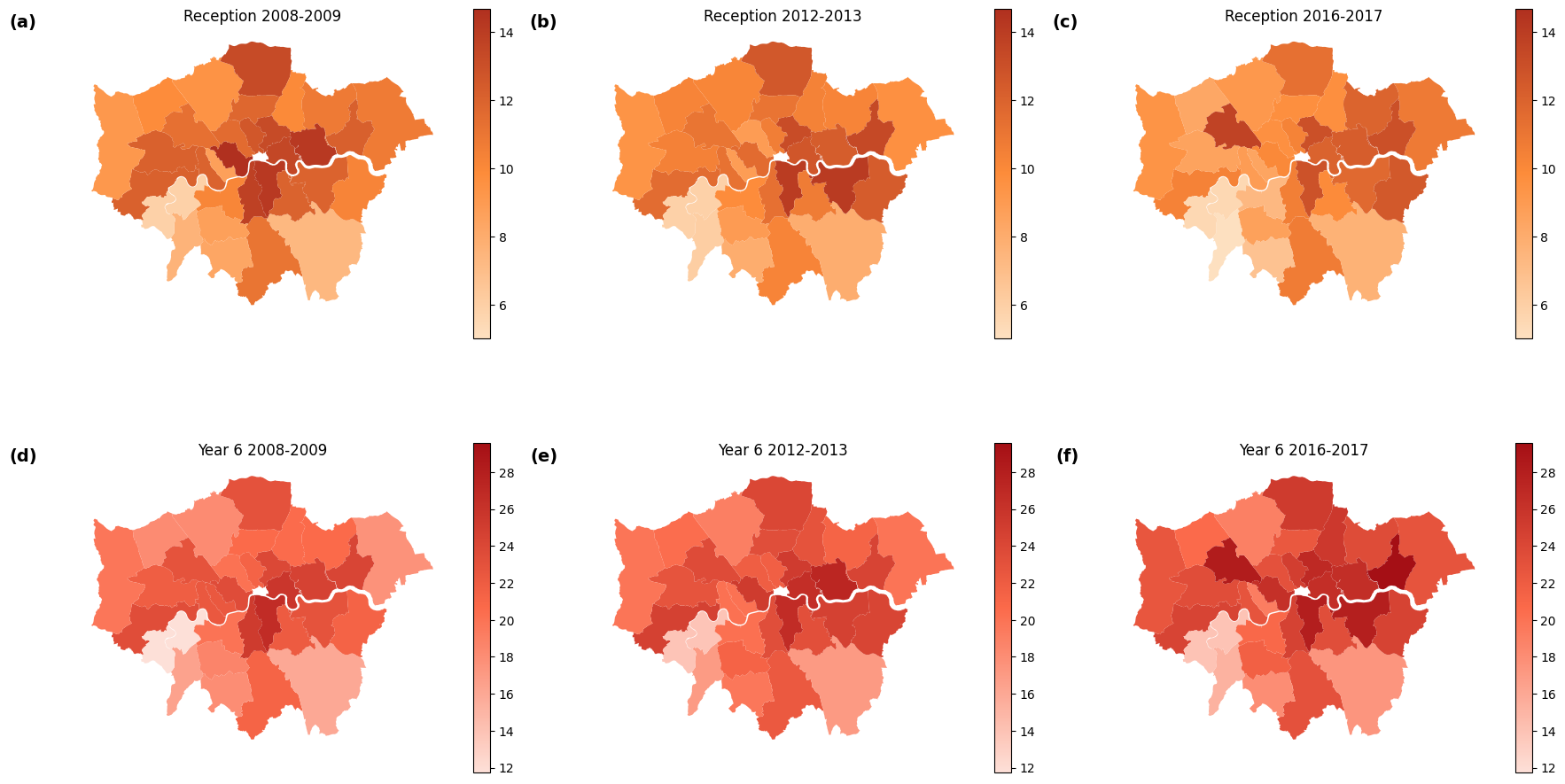
Nowadays, childhood obesity has become an increasingly significant public health challenge, particularly in developed urban environments. In order to have a better understanding of this heated topic, this study investigates the obesity rate of children in London by analyzing its spatial and temporal trends. Besides, the research uses multiple linear regression to examine the possible socioeconomic driving factors of childhood obesity, including poverty rates, sports participation, crime rates, and the number of looked-after children (LAC). The findings reveal that the obesity rate of year 6 (ages 10-11) children showed an upward trend from 2008 to 2018, and eastern London boroughs had a relatively high childhood obesity rate during this period. As for the driving factors, poverty rates have the strongest correlation with the childhood obesity rate, followed by sports participation rates, while crime rates and LAC rates show weaker associations. The results highlight the need for targeted public health interventions addressing socioeconomic disparities and promoting physical activity to reduce childhood obesity in high-risk areas, particularly in eastern London boroughs.

 View pdf
View pdf


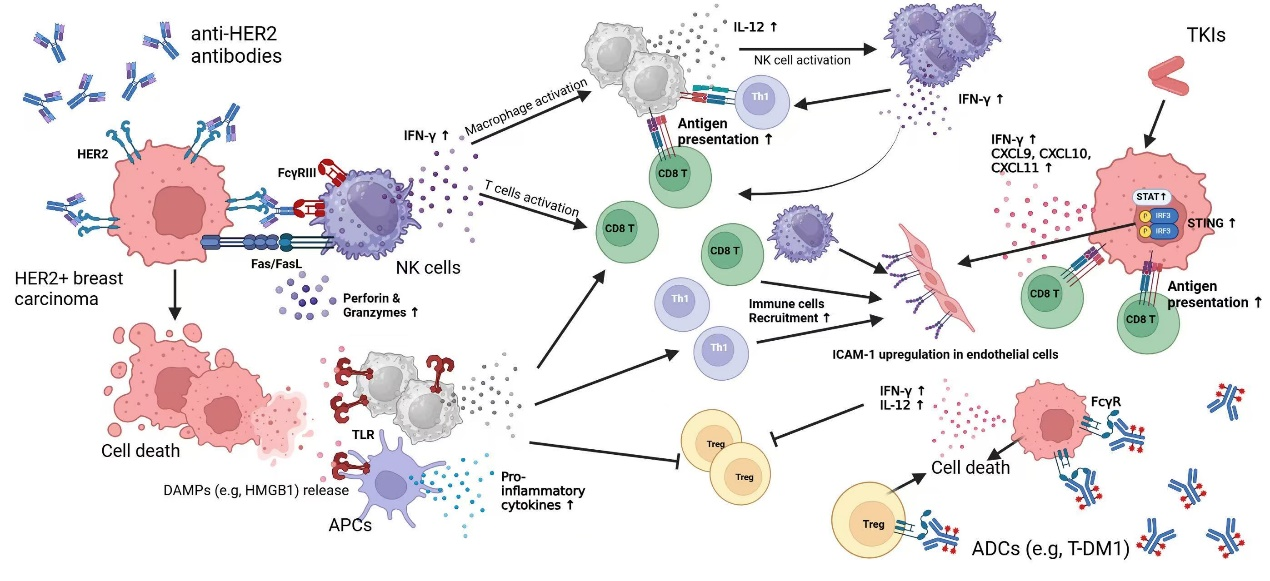
Breast cancer is a heterogeneous malignancy that consists of multiple molecular subtypes, each requiring therapeutic approaches distinctively. While traditional treatments, including endocrine therapy, and anti-HER2 therapy, primarily targeting on cancer cell proliferation and survival, emerging evidence underscores their profound immunomodulatory effects within the tumor microenvironment. The intricate interplay between breast cancer treatments and immune cells shapes therapeutic efficacy and patient outcomes. This review aiming to analysis the immunomodulatory functions of various therapies, including selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), and CDK4/6 inhibitors; monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and antibody-drug conjugates (ADCs); and the role of PARP inhibitors, PI3K inhibitors, and mTOR inhibitors in modulating immune responses. We discuss how these treatments impact key immune populations, including tumor-infiltrating lymphocytes, myeloid-derived suppressor cells (MDSCs), and natural killer (NK) cells, as well as their influence on immune checkpoints and cytokine signaling pathways. Understanding the dual role of breast cancer therapies in both suppressing tumor growth and modulating the immune landscape offers new opportunities for strategies integrating targeted therapy and immunotherapy. By elucidating these interactions, we aim to provide insights into optimizing treatment strategies to enhance antitumor immunity and improve patient prognosis.

 View pdf
View pdf


With the development of technology, although it has become easier to detect and treat oral cancer, it still causes significant harm to people’s lives. Therefore, it is urgent for researchers to clarify the mechanisms behind oral cancer and identify early detection signatures. Previous research has shown a connection between bacteria and the occurrence of cancer detected in other human organs, such as the intestinal tract and stomach. Subsequently, the potential principles of how oral bacteria increase the happening of oral cancer has attracted considerable interest from researchers. This article briefly introduces different signatures of oral bacteria in various human body conditions, such as variations in distribution and composition. Additionally, this passage summarizes the latest research results about the relationship between bacteria and the occurrence of oral cancer.

 View pdf
View pdf


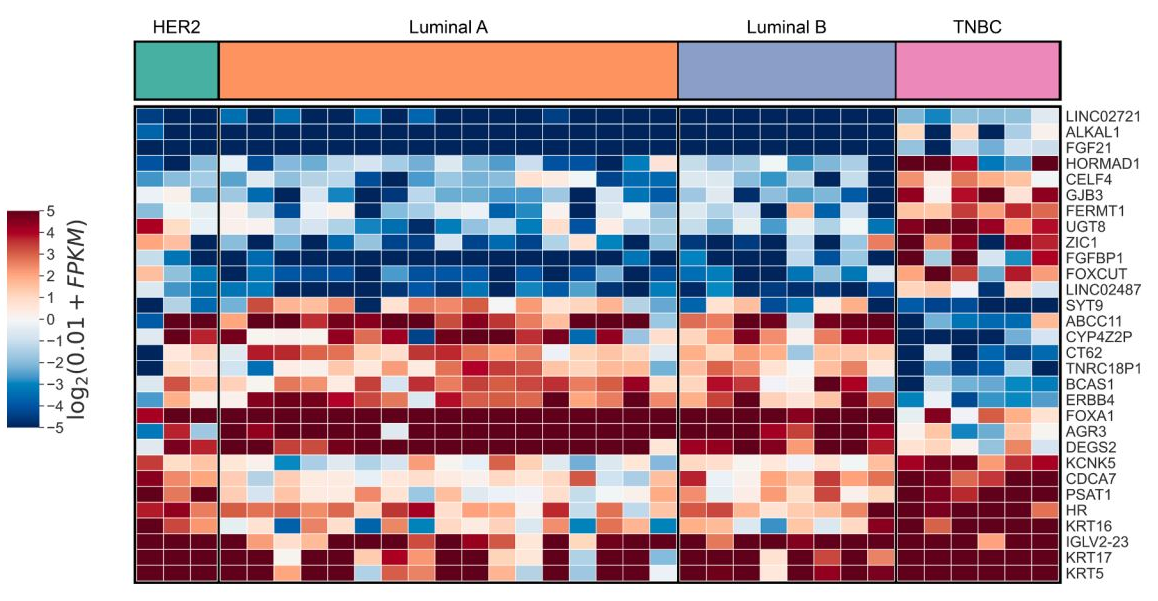
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer and a leading cause of cancer-related mortality in women. The incidence of TNBC is particularly high in China, yet treatment options remain limited, with chemotherapy—often associated with severe side effects—being the primary therapeutic approach. This study investigates the role of LINC02487 in TNBC through in vitro experiments to assess its potential as a biomolecular marker for TNBC treatment. RT-qPCR analysis revealed that LINC02487 expression was significantly upregulated in TNBC. Lentiviral transfection was used to establish LINC02487 overexpression cell lines. CCK-8 assays and EdU staining demonstrated that LINC02487 overexpression promoted TNBC cell proliferation at 24, 48, and 72 hours. Conversely, knockdown of LINC02487 inhibited TNBC cell proliferation, as confirmed by experiments using LINC02487 knockdown cell lines. Furthermore, Transwell assays indicated that LINC02487 knockdown suppressed TNBC cell migration and invasion. Overall, this study confirms the high expression of LINC02487 in TNBC and demonstrates that its knockdown can inhibit cell proliferation and metastasis, thereby slowing TNBC progression. These findings suggest that LINC02487 has potential as a novel prognostic marker and therapeutic target for TNBC, offering new hope for improved patient outcomes.

 View pdf
View pdf


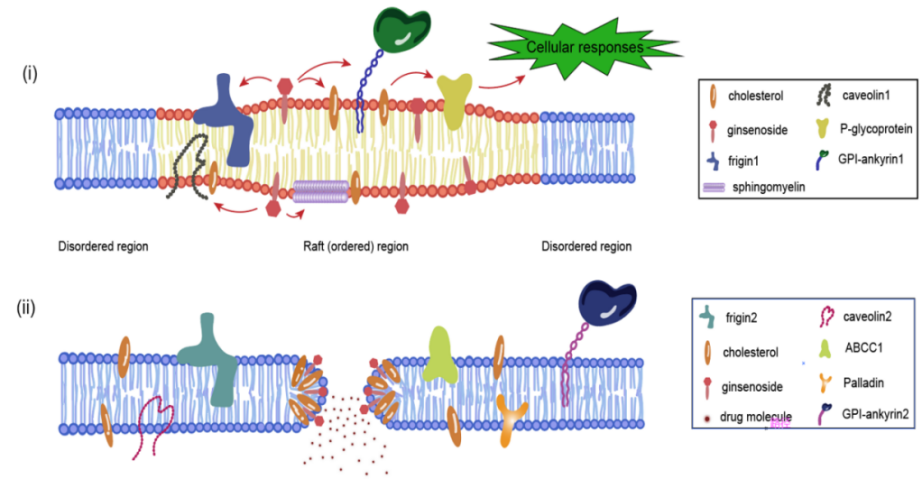
Permeation properties of biofilms have been the theme and hotspot of natural medicinal chemistry research. Ginsenoside is the main active ingredient of ginseng and a membrane active compound that has been used as a drug and surfactant for a long time. The unique amphiphilic structure of ginsenoside determines that it has a hydrophobic triterpene or steroid structure and a hydrophilic glycosyl portion, thus exhibiting potent membrane-disrupting activities such as hemolytic activity, whereas the effect of the activity is dependent on the core structure of the steroid or triterpene and on the sugar chain. Therefore, we are interested in the application of ginsenosides as drug carriers. When the concentration exceeds the critical micelle concentration, ginsenosides can interact with biofilms and aggregate on the membrane to form transient pores, thereby increasing membrane permeability and fluidity. In this paper, the research progress of ginsenoside-membrane interaction and the green application of ginsenoside as a drug carrier in recent years are summarized, with a view to providing a good prospect for the development of ginsenoside for clinical application.

 View pdf
View pdf


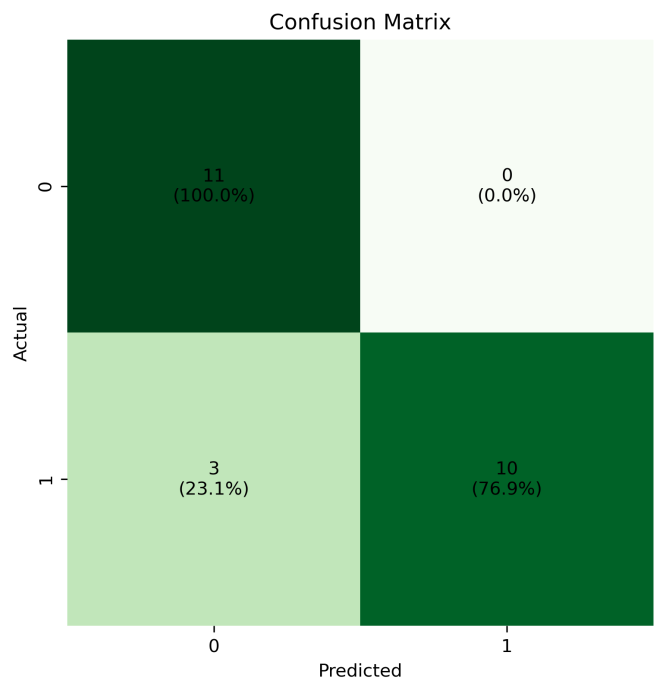
In this study, an XGBoost model optimized based on the Newton-Raphson algorithm is proposed for the task of kidney stone risk prediction, which improves the parameter updating strategy of the traditional gradient boosting framework by introducing second-order derivative information. In order to verify the effectiveness of the model, the performance differences between decision trees, random forests, standard XGBoost, CatBoost and the optimized model are systematically compared. The experimental results show that the XGBoost model optimized by the Newton-Raphson algorithm reaches 0.875 in both accuracy and recall indexes, which is significantly better than the other compared models, and its balanced assessment indexes both reflect the accurate identification ability of positive samples and verify the reliability of the overall prediction performance. Particularly noteworthy is that although Random Forest and standard XGBoost perform consistently in accuracy and recall, the differences in precision rate and AUC value reveal the essential difference between the two in feature space division and integration strategy: Random Forest reduces the risk of overfitting through feature randomness, while XGBoost relies on the regularization term to control the model complexity. The research results not only confirm the feasibility of the optimization algorithm in improving the performance of medical prediction models, but also provide an intelligent tool with practical application value for early screening and risk assessment of kidney stones in clinical practice with its stable prediction accuracy of 0.875.

 View pdf
View pdf




